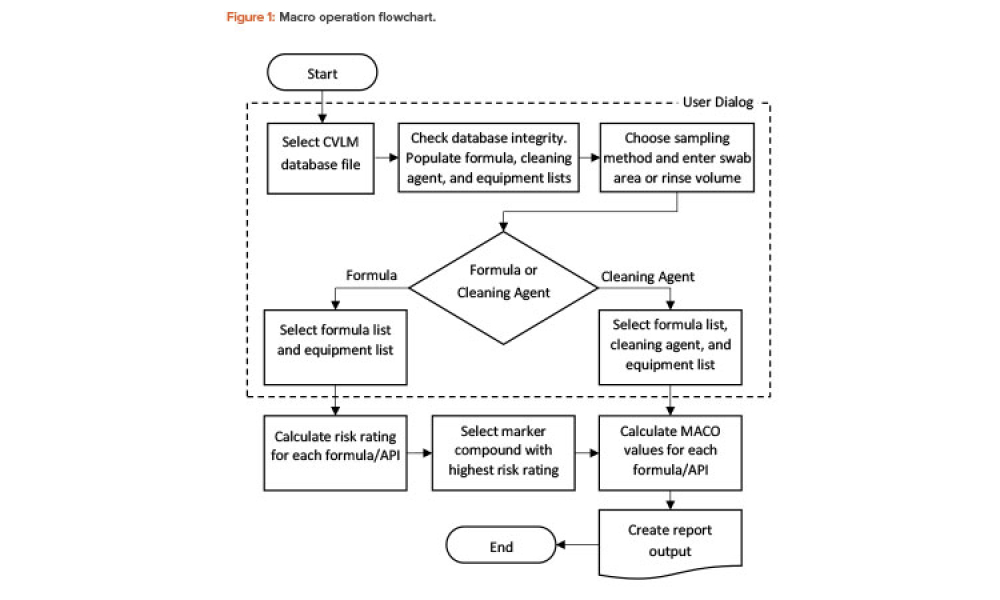
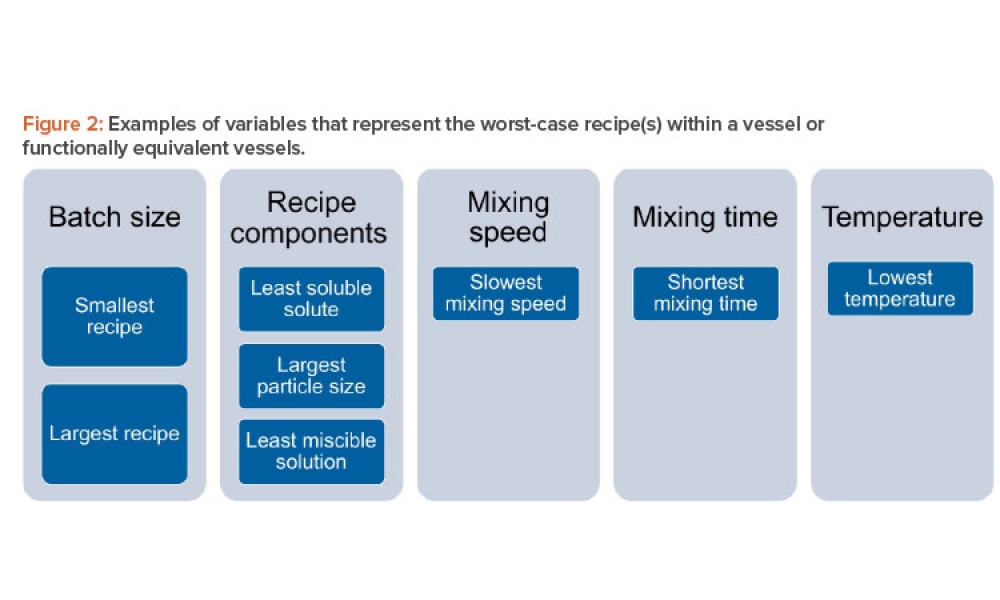
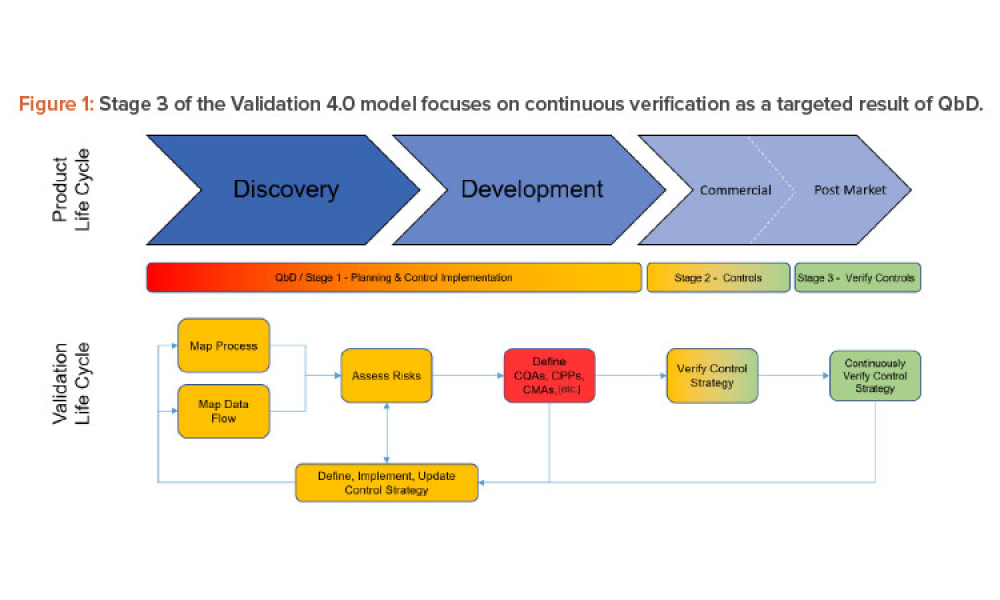
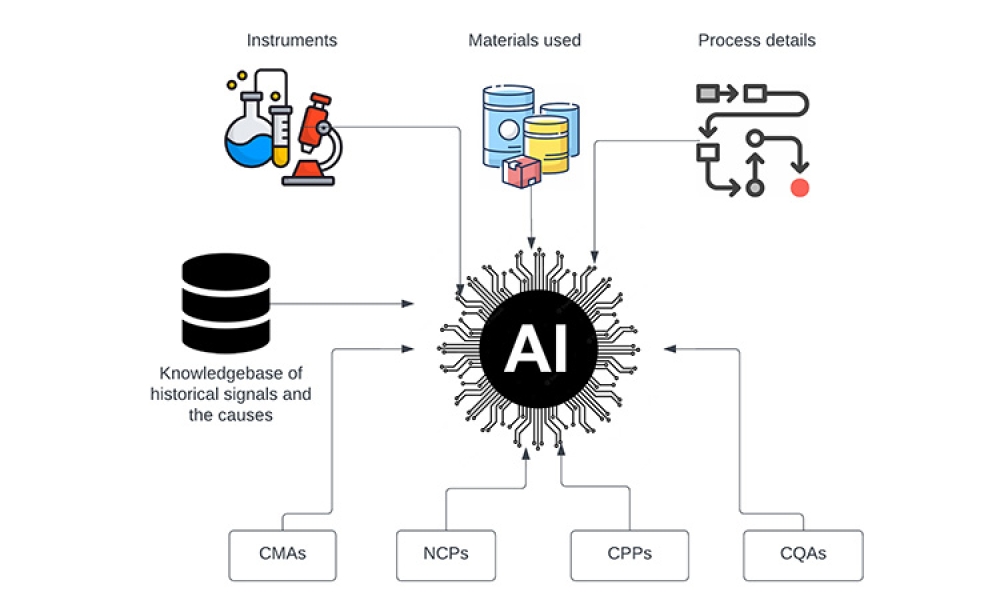
Validation is creating an evidence trail to show that an action, method, or system leads to a consistent and reproducible result. Validation is the collection and evaluation of data from the process design stage through commercial production, which establishes scientific evidence that a process or components of a process can consistently deliver a quality product. Process validation involves a series of activities taking place over the lifecycle of the product and process.
Guidance Documents
Data Integrity (11)
+GAMP® (12)
+Lifecycle Management (1)
+Microbiological & Viral Contamination Control (1)
+Quality Assurance (1)
+Validation (14)
+Community Discussions
Community Discussions
Apr 16, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Apr 08, 2025
Validation
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Feb 03, 2025
Jan 27, 2025
Dec 04, 2024
GAMP®
Lifecycle Management
Oct 31, 2024
Regulatory
Quality
Good Manufacturing Practice
Sustainable Facilities, HVAC, & Controlled Environments
Pharmaceutical Engineering Magazine Articles
Webinars
Upcoming
On-Demand
White Papers
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…
Lifecycle Approach to Biotech Process Validation
This discussion paper proposes ideas for answering the questions about the application of the…
Stage 3 Process Validation: Applying Continued Process Verification Expectations
This discussion paper proposes ideas for answering the questions “How is Stage 3 monitoring and…

Pharmaceutical Job Board
Videos
iSpeak Blog Posts
Featured Conferences