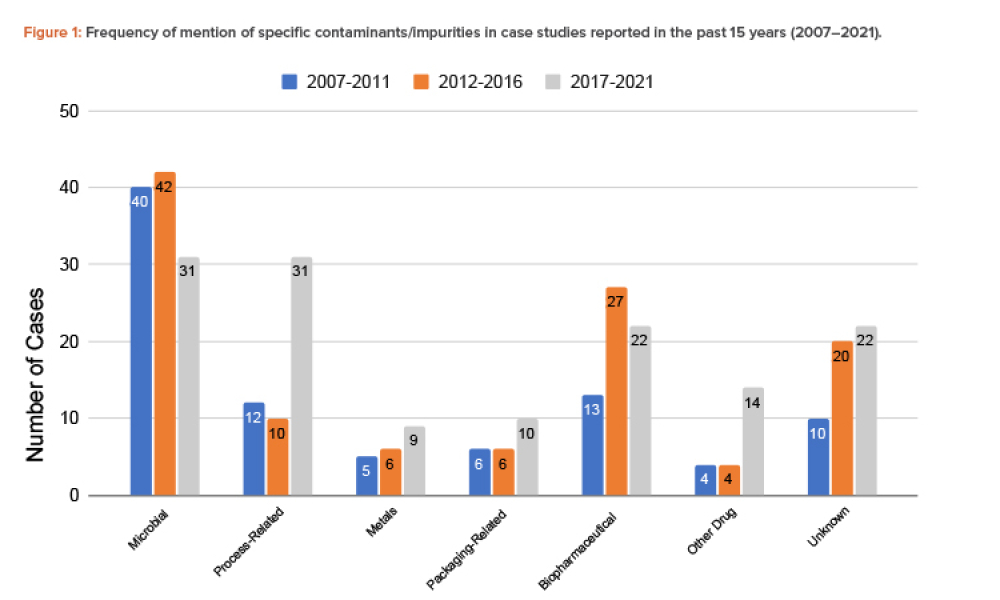
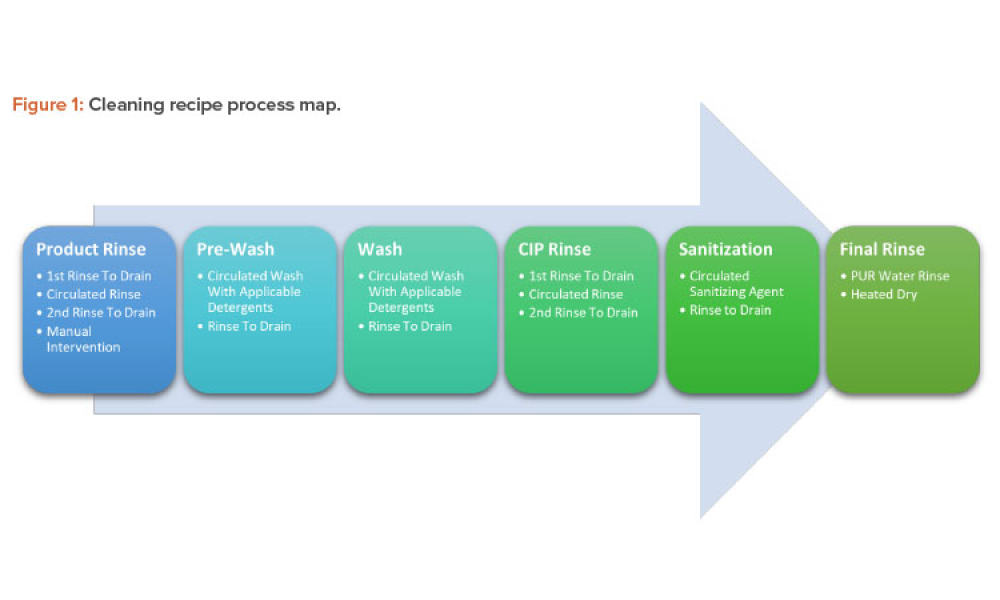
Quality Assurance (QA): Pharmaceutical quality assurance may be defined as the sum of all activities and responsibilities required to ensure that the medicine that reaches the patient is safe, effective, and acceptable to the patient.
Guidance Documents
Compounding (1)
+Drug Shortages (1)
+Good Manufacturing Practice (1)
+Knowledge Management (4)
+Project Management (1)
+Quality Assurance (8)
+Validation (1)
+Community Discussions
Community Discussions
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Nov 04, 2024
Information Systems
Management
Pharma 4.0™
Project Management
Nov 04, 2024
Regulatory
Supply Chain
Active Pharmaceutical Ingredients
Good Manufacturing Practice
Oct 31, 2024
Regulatory
Quality
Good Manufacturing Practice
Sustainable Facilities, HVAC, & Controlled Environments
Pharmaceutical Engineering Magazine Articles
Webinars
Upcoming
On-Demand
White Papers
September / October 2023
Regulatory Trends & Quality Initiatives - Shortages of essential medicines around the world have…
July / August 2022
ISPE held an Expert Xchange on 18 January 2022 that included presentations and interactive exercises…