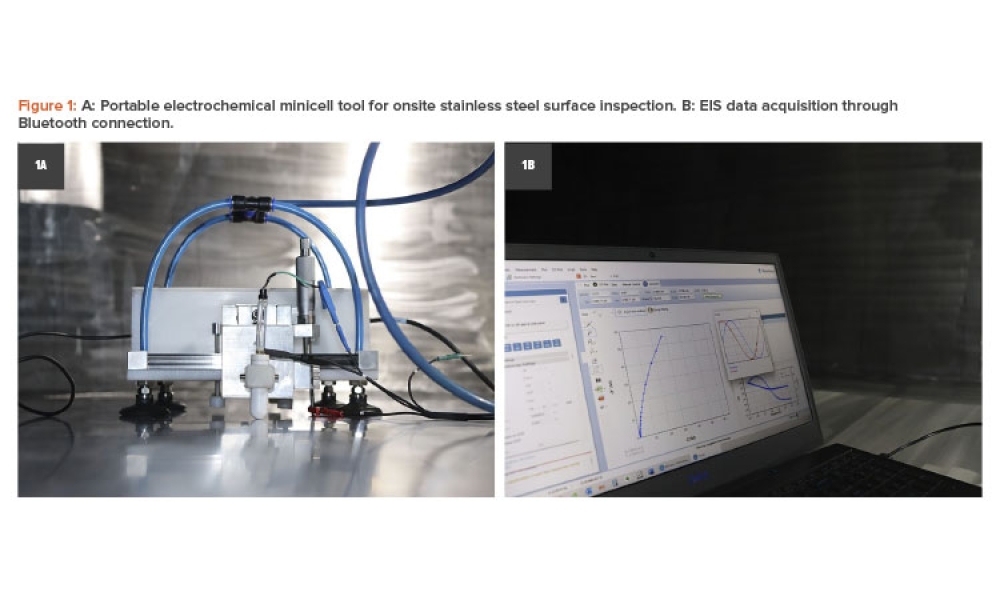
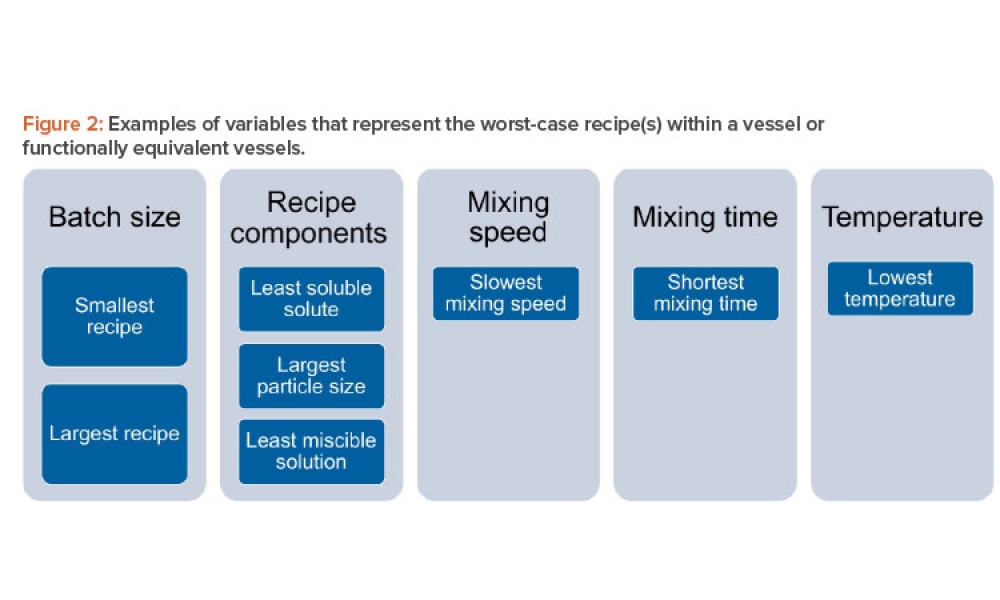
Content focuses on the role and elements of a quality management system, its impact on the overall risk management approach, and its implementation scientifically and pragmatically. Case studies could demonstrate risk management's purpose, elements, and implementation, a quality management system, and systems validation.
Guidance Documents
Biotechnology (1)
+Commissioning & Qualification (4)
+Compounding (1)
+Containment (1)
+Data Integrity (15)
+Drug Shortages (1)
+GAMP® (15)
+Good Manufacturing Practice (1)
+Knowledge Management (6)
+Lifecycle Management (3)
+Manufacturing Operations (1)
+Microbiological & Viral Contamination Control (2)
+Pharma 4.0™ (1)
+Process Analytical Technology (4)
+Project Management (1)
+Quality Assurance (8)
+Quality Control (1)
+Quality by Design (4)
+Regulatory (2)
+Sustainable Facilities, HVAC, & Controlled Environments (1)
+Validation (13)
+Community Discussions
Community Discussions
Apr 02, 2025
Data Integrity
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Mar 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Mar 20, 2025
Mar 20, 2025
Mar 20, 2025
Data Integrity
Pharmaceutical Engineering Magazine Articles
Webinars
Upcoming
On-Demand
ISPE in the News
Latest
-
ISPE Training: Process Validation Training Course, 8 - 10 April
-
Discover Exciting Career Opportunities on ISPE's Job Board
-
Steele Appointed Acting Director of US FDA's CBER
-
US FDA to Cut Back on Routine Inspections
-
Tariff Threats Create Uncertainty in Pharma Market
-
Expecting the Unexpected Ensures Continuity in Biopharma Manufacturing
-
Digital Twin may Streamline Gene Therapy Production
-
AMT Designation Granted to Cellares' Cell Shuttle
-
Innovative Culture Media Support Intensified Processes
- Navigating Category 3 Compounding Challenges
White Papers
May / June 2024
The commercialization of personalized medicine has ushered in demand for a new type of facility—…
January / February 2024
Stakeholders across industries are becoming accustomed to using information technology (IT) systems…
September / October 2023
Regulatory Trends & Quality Initiatives - Shortages of essential medicines around the world have…
July / August 2023
Continuous manufacturing (CM) challenged regulators’ expectations and regulatory frameworks. This…
May / June 2023
North Carolina’s Research Triangle is the largest of its kind in the US. Thanks to years of effort…