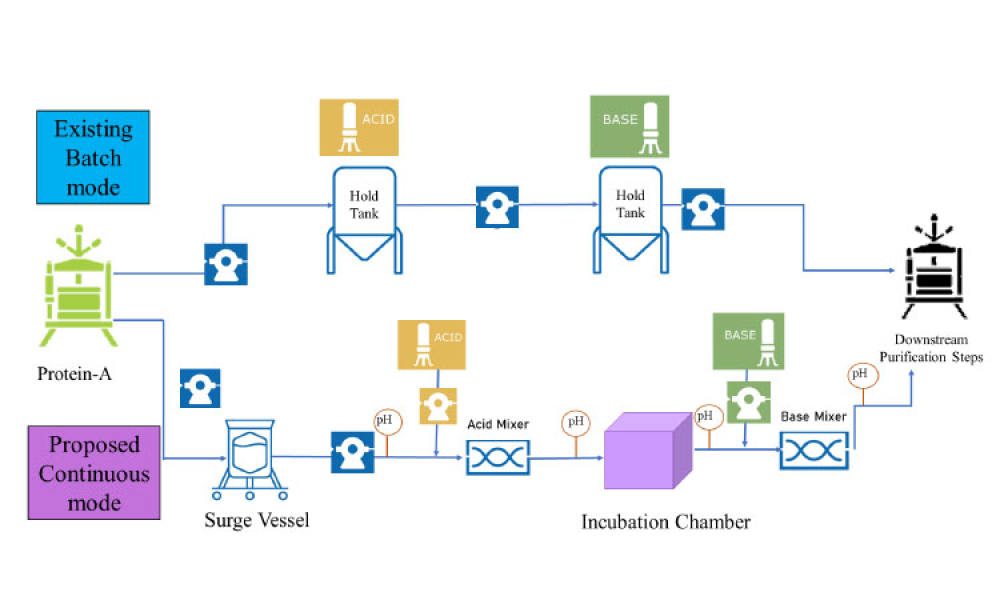
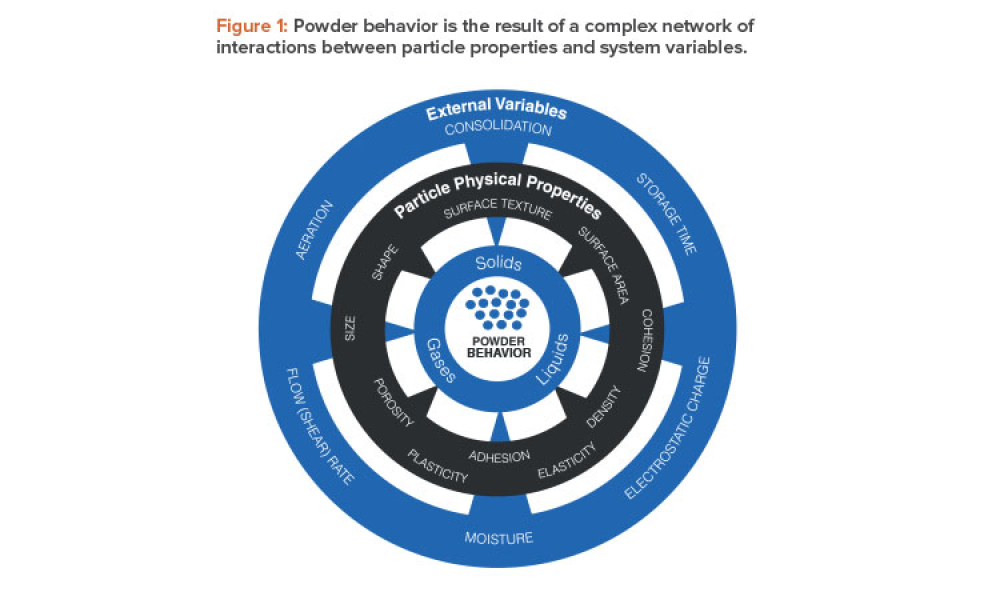
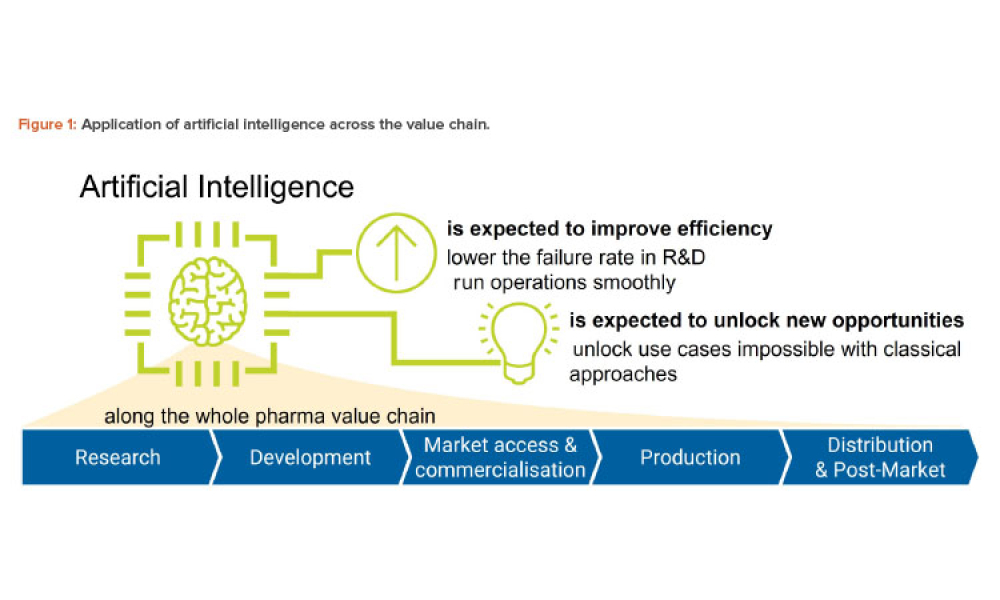
Pharmaceutical Quality by Design (QbD) is a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and control based on sound science and quality risk management.
Guidance Documents
Biotechnology (1)
+Commissioning & Qualification (1)
+Containment (1)
+Lifecycle Management (2)
+Microbiological & Viral Contamination Control (1)
+Process Analytical Technology (2)
+Quality by Design (5)
+Regulatory (2)
+Community Discussions
Community Discussions
Apr 16, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Dec 04, 2024
GAMP®
Lifecycle Management
Oct 23, 2024
Quality
Active Pharmaceutical Ingredients
Good Manufacturing Practice
Pharmaceutical Engineering Magazine Articles
iSpeak Blog Posts
Professional Development Training
First Principles to Improve Pharma Manufacturing Operations Training Course
+Process Validation Training Course
+Turning Quality by Design (QbD) into a Practical Reality Training Course
+Facilities Management Training Course
+White Papers
July / August 2024
The explosive growth of advanced therapy medicinal products (ATMPs), particularly cellular…
May / June 2024
The commercialization of personalized medicine has ushered in demand for a new type of facility—…
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…