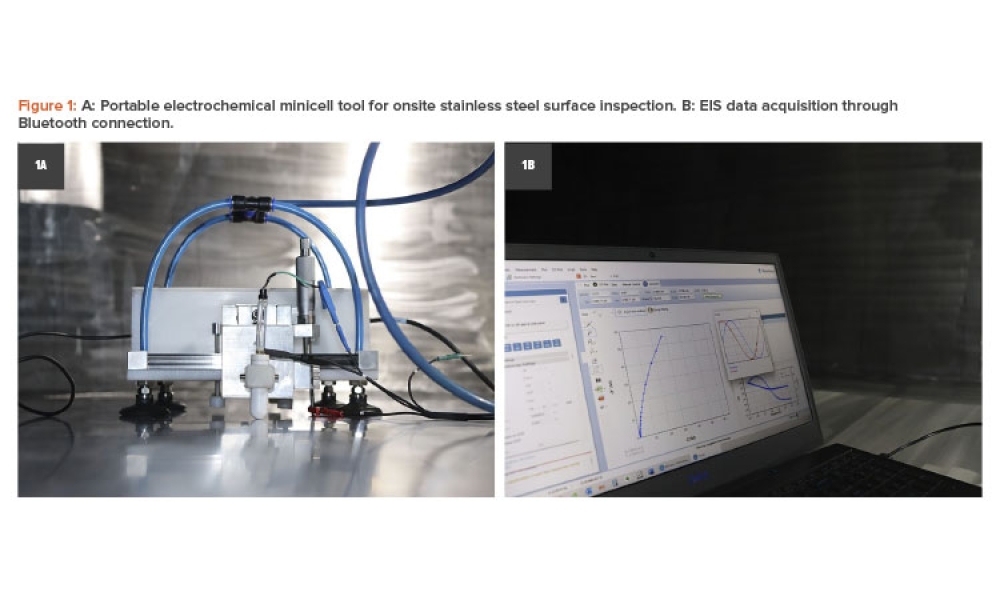
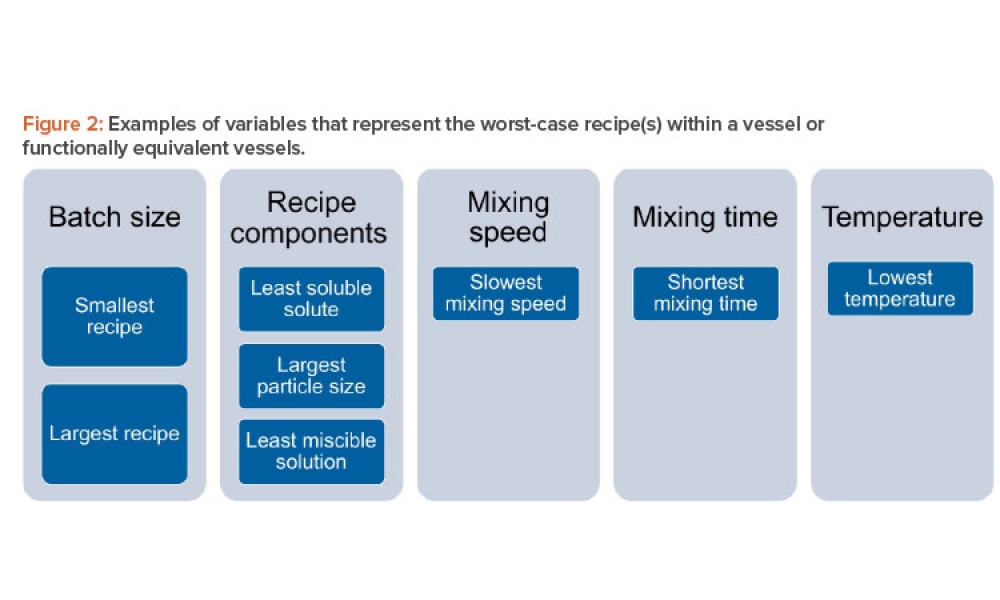
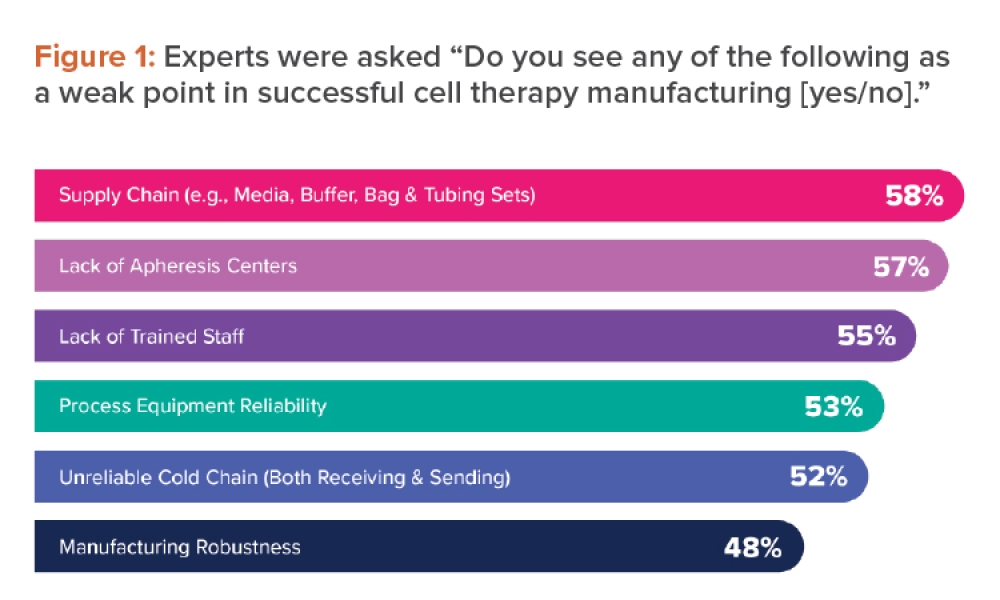
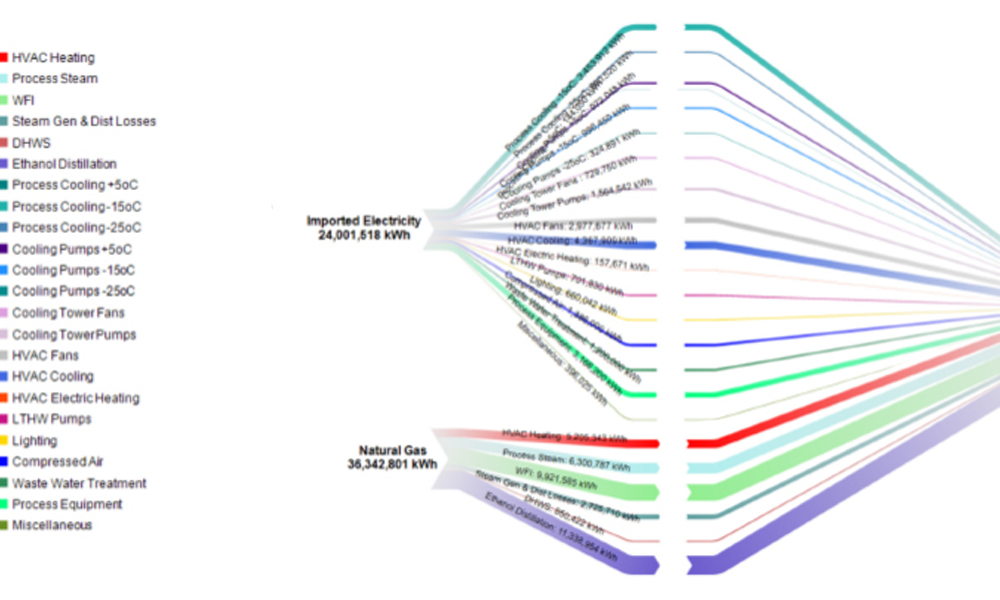
Content focuses on process equipment, the design of facilities, and utility systems that support the critical physical and chemical requirements of drug products, in addition to other aspects of the product specification. Topics include design and construction/installation, commissioning and qualification, operation and maintenance, controls, and automation.
Related Guidance Documents
Advanced Manufacturing (3)
Advanced Therapy Medicinal Products (1)
Biotechnology (3)
Commissioning & Qualification (4)
Compounding (2)
Containment (4)
Critical Utilities (8)
- Good Practice Guide: Process Gases 2nd Edition
- Baseline Guide Vol 4: Water & Steam Systems 3rd Edition
- Good Practice Guide: C&Q of Pharma Water & Steam Systems 2nd Edition
- Good Practice Guide: Ozone Sanitization of Pharma Water Systems
- Good Practice Guide: Membrane-Based WFI Systems
- Good Practice Guide: Critical Utilities GMP Compliance
- D/A/CH Affiliate: WFI Handbook (English Translation)
- Good Practice Guide: Sampling Pharma Water, Steam, & Process Gases
Data Integrity (2)
Good Manufacturing Practice (1)
Manufacturing Operations (8)
- Guide: ATMPs - Allogeneic Cell Therapy
- Baseline Guide Vol 2: Oral Solid Dosage Forms 3rd Edition
- Good Practice Guide: Continuous Manufacturing of Oral Solid Dosage Forms
- Guide: ATMPs - Autologous Cell Therapy
- GAMP Good Practice Guide: Manufacturing Execution Systems
- Good Practice Guide: Maintenance 2nd Edition
- Good Practice Guide: Equipment Reliability
- Baseline Guide Vol 3: Sterile Product Manufacturing Facilities 3rd Edition
Microbiological & Viral Contamination Control (16)
- Good Practice Guide: Process Gases 2nd Edition
- Baseline Guide Vol 2: Oral Solid Dosage Forms 3rd Edition
- Baseline Guide Vol 4: Water & Steam Systems 3rd Edition
- Guide: Cleaning Validation Lifecycle - Applications, Methods, & Controls
- Good Practice Guide: Quality Lab Facilities
- Good Practice Guide: Ozone Sanitization of Pharma Water Systems
- Good Practice Guide: Membrane-Based WFI Systems
- Good Practice Guide: Heating, Ventilation, & Air Conditioning (HVAC)
- Good Practice Guide: Assessing Particulate Containment 2nd Edition
- Good Practice Guide: Containment for Potent Compounds
- D/A/CH Affiliate: WFI Handbook (English Translation)
- Good Practice Guide: HVAC & Process Equipment Air Filters
- Good Practice Guide: Asset Management
- Baseline Guide Vol 3: Sterile Product Manufacturing Facilities 3rd Edition
- Japan Affiliate: Pest Control Manual (English Translation)
- Good Practice Guide: Sampling Pharma Water, Steam, & Process Gases
Oral Solid Dosage (2)
Pharma 4.0™ (1)
Quality Assurance (1)
Quality Control (2)
Quality by Design (2)
Regulatory (3)
Single Use Technologies & Disposables (1)
Sterile Products (1)
Supply Chain Management (2)
Sustainability (1)
Sustainable Facilities, HVAC, & Controlled Environments (6)
- Good Practice Guide: Cold Chain Management
- Good Practice Guide: Heating, Ventilation, & Air Conditioning (HVAC)
- Good Practice Guide: Assessing Particulate Containment 2nd Edition
- Good Practice Guide: Containment for Potent Compounds
- Good Practice Guide: Controlled Temperature Chambers 2nd Edition
- Good Practice Guide: HVAC & Process Equipment Air Filters
Community Discussions
Community Discussions
Dec 20, 2024
Dec 19, 2024
GAMP®
Dec 18, 2024
Dec 16, 2024
Biotechnology
Containment
Dec 11, 2024
Data Integrity
Dec 04, 2024
Good Manufacturing Practice
Dec 04, 2024
GAMP®
Lifecycle Management
Pharmaceutical Engineering Magazine Articles
Webinars Related to Facilities & Equipment
Concept and Discussion Papers
Pharma 4.0 – Towards IT/OT Architectures for Prescriptive Maintenance
Introduction Faced with global competition and shrinking margins, (bio)pharmaceutical manufacturers…
Process Events for the Life Science Industry - Information Model Concept on OPC UA for Alarms and Audit Trails
Abstract Plug & play in general describes a piece of equipment that is ready to use upon connecting…
July / August 2023
Continuous manufacturing (CM) challenged regulators’ expectations and regulatory frameworks. This…
May / June 2023
North Carolina’s Research Triangle is the largest of its kind in the US. Thanks to years of effort…
A Simplified Integration of Qualified Laboratory Devices with the Asset Administration Shell as the Digital Twin
Abstract Plug & play in general describes a piece of equipment that is ready to use upon connecting…

Videos Related to Facilities & Equipment
iSpeak Blog Posts Related to Facilities & Equipment
Training Courses Related to Facilities & Equipment
GMP Fundamentals: Eleven-Part Bundle Series
Obtain a 10% Savings by Purchasing All Eleven Courses Overview ISPE is presenting an eleven-part series that will focus on the fundamentals of good manufacturing practices (GMPs). The series provides an overview of the regulations pertaining to GMPs and covers topics such as: manufacturing controls, product distribution, plant hygiene, documentation practices, buildings & facilities, organizational structure, and more. This offering gives users access to all eleven modules of the series and is intended to introduce GMPs for the new pharmaceutical employee or to provide an annual refresher for…
Biopharmaceuticals: Webinar C> e ATMP
Biopharmaceuticals: Webinar de treinamento em C> e ATMP Visão geral Este webinar é uma gravação do novo curso de instrutor que é um curso de nível avançado sobre therapies C e Gene T (C>) e Medicamentos de Terapia Avançada (ATMP). O curso também fornece umavisão geral dos componentes mais comuns e estabelecidos que são aproveitados em produtos C> (por exemplo, plasmídeos, mRNA, nanopartículas lipídicas, vetores virais), cobrindo terminologia, processos de fabricação e subsequente caracterização analítica de os componentes fabricados e os produtos terapêuticos. As plataformas de células T…
Produtos Biofarmacêuticos: Aspectos CMC
Modo de Entrega: Webinar Produtos Biofarmacêuticos: Webinar de Treinamento sobre Aspectos CMC Visão geral Este webinar é uma gravação do novo curso de instrutor, que é um curso de nível avançado sobre aspectos de Química, Fabricação e Controle (CMC) do desenvolvimento biofarmacêutico. O objetivo deste curso é fornecer uma compreensão avançada dos aspectos CMC de biofármacos desde o desenvolvimento até a comercialização. O que você vai aprender Visão geral do desenvolvimento biofarmacêutico Qualidade por Design (Quality by Design – QbD) Linhagem Celular e Desenvolvimento a Montante (Upstream)…
GMP Fundamentals: Packaging & Labeling Control
In this course, you will learn how to ensure the safety and quality of pharmaceutical products using fundamental principles and best practices. During this course, you will learn about the FDA's pharmaceutical packaging and labeling guidelines. You will gain an understanding of the basics of packaging and labeling operations, including production and inspection processes, documentation, and record-keeping. Interactive Course: What to Expect CEUs are provided once you achieve an 80% passing grade and complete the evaluation. Buy Now Return to Online Learning
GMP Fundamentals: Buildings and Facilities
A “GMP Facility” is a building that is used in the production of pharmaceutical and medical device products. The proper design, layout, function and control of such facilities is essential in the manufacture of pure, safe and effective pharmaceutical products. In this course, learners will focus on understanding the building layout, safety, cleanroom conditions, as well as facility sanitation and ventilation of both production and support areas. In this On Demand course (part 4 of 11), you will learn the elements of GMP Buildings & Facilities, as required by regulation. Interactive Course…