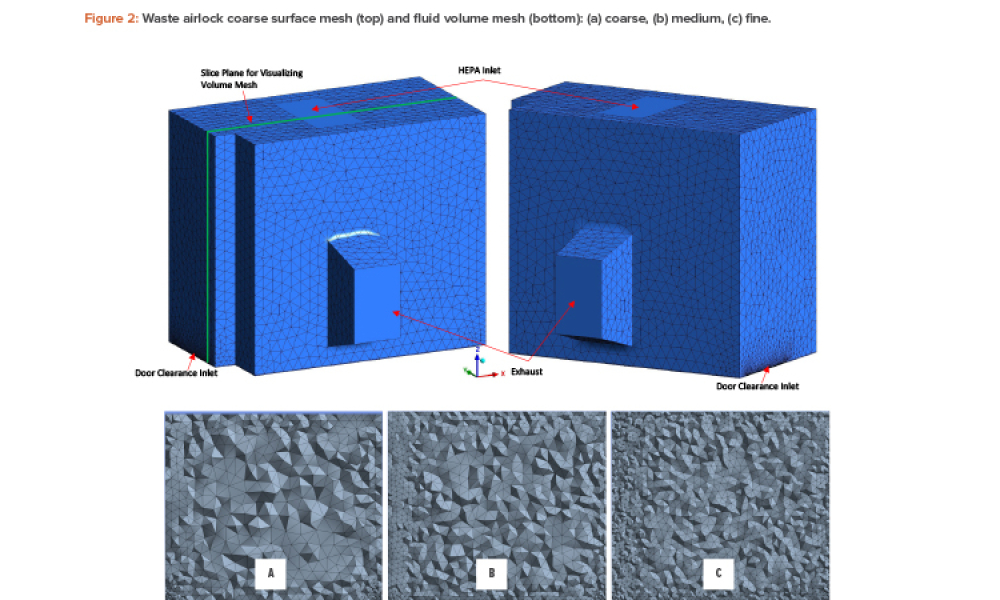
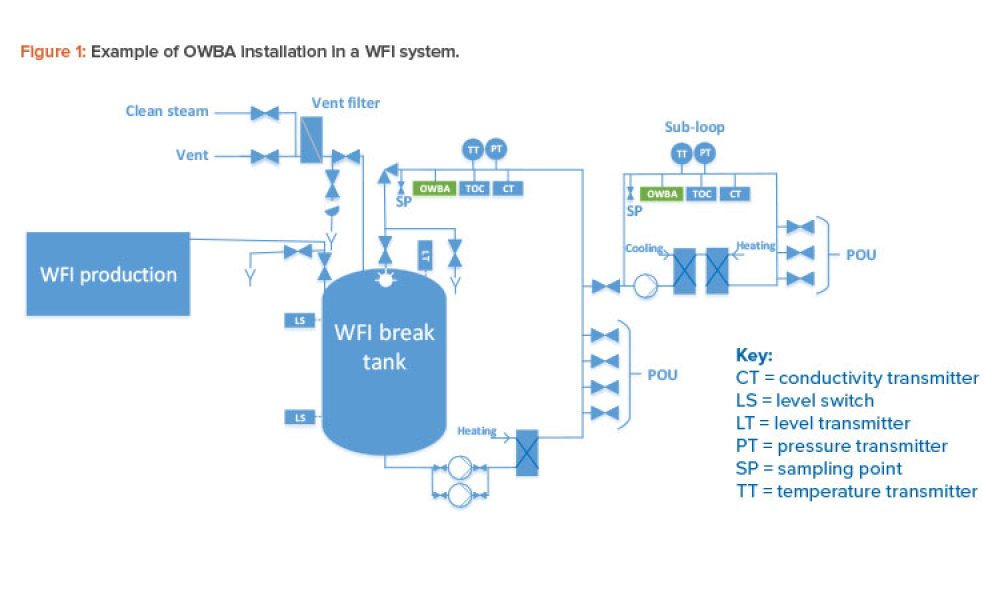
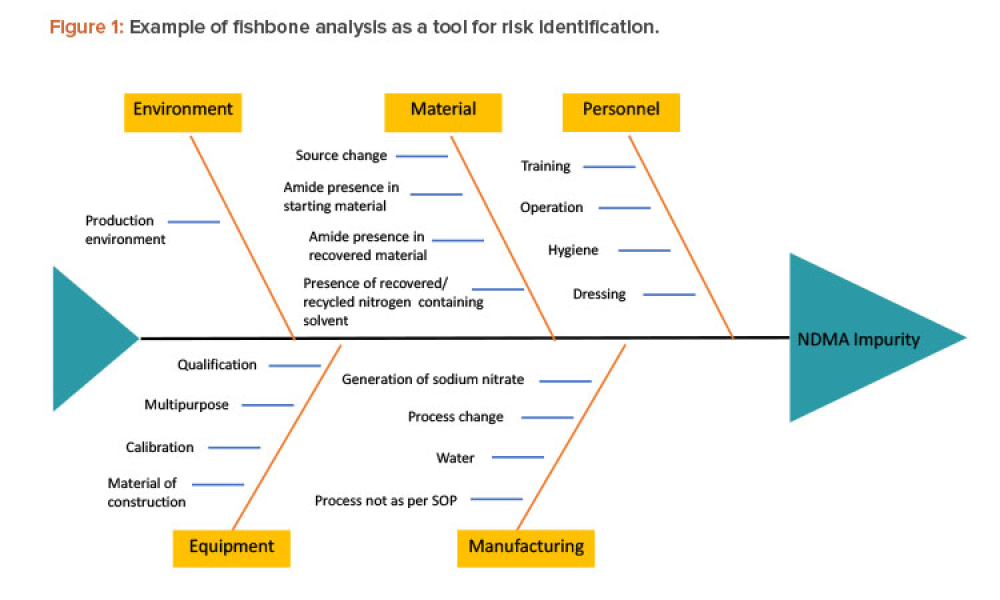
Quality Control (QC) is a detailed system of inspection and control covering the production, evaluation, and distribution of pharma drugs a manufacturer produces.
Guidance Documents
Critical Utilities (1)
+Microbiological & Viral Contamination Control (2)
+Quality Control (2)
+Community Discussions
Community Discussions
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Nov 04, 2024
Regulatory
Supply Chain
Active Pharmaceutical Ingredients
Good Manufacturing Practice
Oct 31, 2024
Regulatory
Quality
Good Manufacturing Practice
Sustainable Facilities, HVAC, & Controlled Environments
Oct 23, 2024
Quality
Active Pharmaceutical Ingredients
Good Manufacturing Practice
Webinars
Upcoming
On-Demand
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
ICH Q6A: Specifications, Test Procedures, and Acceptance Criteria for New Drug Substances and Products
+GMP Fundamentals for the Pharmaceutical Industry
+GMP Auditing for Quality Assurance Training Course
+White Papers
July / August 2024
The explosive growth of advanced therapy medicinal products (ATMPs), particularly cellular…
May / June 2024
The commercialization of personalized medicine has ushered in demand for a new type of facility—…
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…