The Asia Pacific region (APAC), like any large territory, encompasses a blend of well-established and early-stage economies, diverse healthcare systems, and differences in language, culture, politics, and technology adoption. APAC’s size and complexity has created new challenges and opportunities for the pharmaceutical industry as nations work together to meet the manufacturing needs for...

Downloads
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact
Cover: The Asia Pacific region (APAC), like any large territory, encompasses a blend of well established and early-stage economies, diverse healthcare systems, and differences in language, culture, politics, and technology adoption. APAC’s size and complexity has created new challenges and opportunities for the pharmaceutical industry as nations work together to meet the manufacturing needs for medical products.
Evolving China’s Regulatory System In Alignment With ICH
Feature: With the Chinese government initiating drug regulatory reform in 2015 and China joining the International Council for Harmonisation (ICH) in 2017, a significant number of measures have been implemented by the government. The aim is to make fundamental changes to China’s drug regulatory administration system so it can facilitate pharmaceutical development and better meet patient needs in the country.
Digital Display Labeling in Clinical Supplies for Clinical Trials
Feature: Digital display labels (DDLs) offer an alternative solution to eliminate manual relabeling in the clinical supply chain, optimizing label content updates through a simple, system controlled approach while providing new, uncharted opportunities. With increased efficiency in making regulatory-compliant changes and enhanced flexibility in the clinical supply chain, DDL technology has the potential to revolutionize drug development and to increase and expedite patient access to therapies.
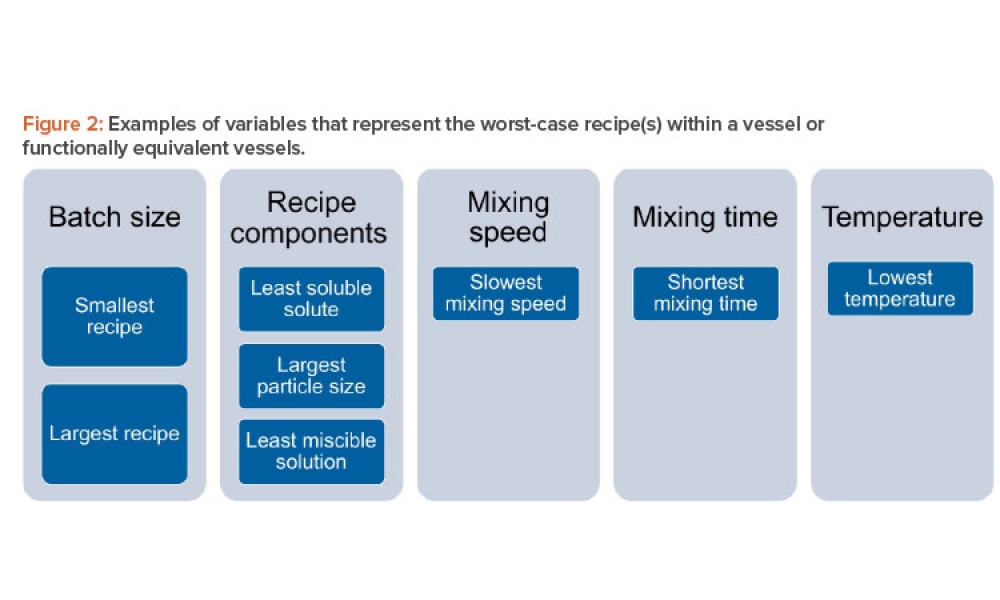
Risk- And Science-Based Media and Buffer Mixing Validations
The validation of media and buffer mixing is a continuing area of resource constraint in the pharmaceutical industry. These validations require materials, validation associates’ time, and the use of equipment and processing areas. This article proposes a risk-based life cycle for minimizing mixing validation resource inputs, with the objective of optimizing validation efforts through the use of recipe bracketing and predictive methods to identify the worst-case recipes that should be validated.
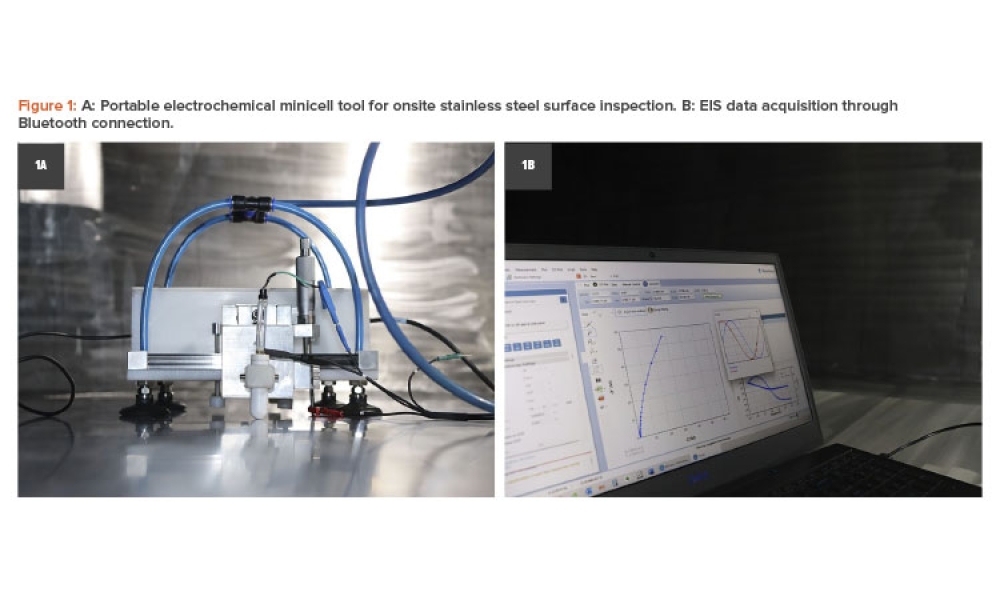
Electrochemical Techniques for Onsite Surface Qualification
Pharmaceutical critical utilities are typically built of 316L stainless steel; nevertheless, surface degradation has been reported due to the occurrence of different phenomena. This article aims to explain how field electrochemical techniques using a portable tool can be an effective method for surface inspection, qualification, and monitoring. The surface finish assessment considered different average roughness, obtained by mechanical polishing and electropolishing, and whether the surface was chemically passivated or not, to generate distinct passive films. This was done to prove the sensitivity of the field electrochemical tool using corrosion techniques.
In This Issue
ISPE has started 2024 with great momentum, having two technical conferences in the first quarter, the 2024 ISPE Facilities of the Future Conference in San Francisco, California and the 2024 ISPE...
Over time and with effort and determination, women in key leadership positions have proven that these positions are genderless for individuals with the correct set of abilities and knowledge.
Personalized medicine provides a treatment alternative that utilizes patients’ genetic material to produce therapeutics. According to Market Research Future, the US currently accounts for the largest share of the personalized medicine market, and it is expected to reach US $27.5 million by 2030.1
The newly released third edition of the ISPE Baseline® Guide: Volume 6 – Biopharmaceutical Manufacturing Facilities reinforces the concepts described in the second...
The impact people have on others’ lives is not always obvious. For many, the Advil mini pill might not seem like a big deal, but for people who have trouble swallowing pills, like cancer patients, the tiny pill has made a huge difference in their ability to manage pain. It’s projects like that, as well as treatments for bladder cancer and transthyretin amyloidosis, that Tammy Spain is most...
Although he didn’t know it at the time, Nathan Temple’s service as a naval officer on the USS Asheville in Hawaii prepared him perfectly for a career in pharmaceutical engineering. “You learn so much, so quickly, on a submarine.”
ISPE has more than 21,000 members in more than 120 countries worldwide. As an ISPE member, you have access to this network, which can be exciting and overwhelming at the same time. Connecting at the local level unlocks the unique benefits that your local Affiliate or Chapter holds. Not only is this a career game changer, but it also opens doors of opportunity to experience your ISPE membership...
The 2023 ISPE Pharma 4.0™ and Annex 1 Conference was held in Barcelona, Spain, 11–12 December. Topics discussed included transforming operations, quality, and maintenance with Pharma 4.0™ principles...
The new year is here and, with that, another International Women’s Day approaches! This incredibly important global movement, which takes place every 8 March, celebrates women’s achievements, raises awareness about discrimination, and advocates for accelerated equality and gender parity. ISPE’s Women in Pharma® community strives to accomplish all these goals through regional and international...
Pharmaceutical critical utilities are typically built of 316L stainless steel; nevertheless, surface degradation has been reported due to the occurrence of different phenomena. This article aims to explain how field electrochemical techniques using a portable tool can be an effective method for surface inspection, qualification, and monitoring. The surface finish assessment considered...
The world is beginning to grasp the huge challenge of achieving net-zero carbon emissions, or carbon neutrality, by 2050. Many countries have committed to achieving this ambitious goal. As a major global industry, the pharmaceutical sector has a significant role to play. For thermal energy–intensive industries, such as pharmaceutical manufacturing, the long-term future options to maintain...
The validation of media and buffer mixing is a continuing area of resource constraint in the pharmaceutical industry. These validations require materials, validation associates’ time, and the use of equipment and processing areas. This article proposes a risk-based life cycle for minimizing mixing validation resource inputs, with the objective of optimizing validation efforts through the use...
In 2022, the US Food and Drug Administration (FDA) issued their draft guidance “Computer Software Assurance for Production and Quality System Software”1
Pharmaceutical manufacturing facilities produce a variety of products, including highly potent products that require safety measures to prevent adverse health effects on patients and operators. To ensure safety, these facilities use containment equipment to minimize the risk of contamination. This article presents criteria for selecting containment equipment, considering both...
Digital display labels (DDLs) offer an alternative solution to eliminate manual relabeling in the clinical supply chain, optimizing label content updates through a simple, system-controlled approach while providing new, uncharted opportunities. With increased efficiency in making regulatory-compliant changes and enhanced flexibility in the clinical supply chain, DDL technology has the...
With the Chinese government initiating drug regulatory reform in 2015 and China joining the International Council for Harmonisation (ICH) in 2017, a significant number of measures have been implemented by the government. The aim is to make fundamental changes to China’s drug regulatory administration system so it can facilitate pharmaceutical development and better meet patient needs in the...