Content focuses on the financial impact of data management systems on drug development, manufacturing, and distribution; the basic computer system life cycle model and the activities and software quality assurance practices in each phase; and the controls and methods necessary to maintain data integrity and security.
Guidance Documents
Advanced Manufacturing (1)
+Commissioning & Qualification (1)
+Data Integrity (15)
+GAMP® (14)
+Knowledge Management (1)
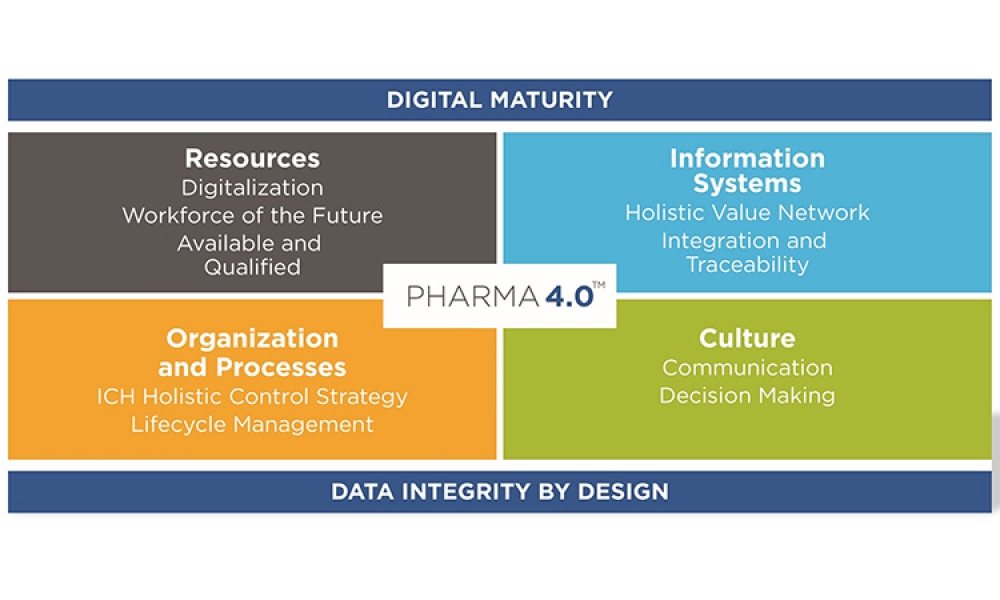
+Pharma 4.0™ (1)
+Regulatory (1)
+Validation (11)
+Community Discussions
Community Discussions
Apr 02, 2025
Data Integrity
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Mar 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Mar 20, 2025
Mar 20, 2025
Mar 20, 2025
Data Integrity
Pharmaceutical Engineering Magazine Articles
White Papers
January / February 2024
Stakeholders across industries are becoming accustomed to using information technology (IT) systems…
March / April 2022
This article aims to refresh information on open-source software (OSS) within regulated computerized…

Webinars
Upcoming
On-Demand
None Available
ISPE in the News
Latest
-
ISPE Training: Process Validation Training Course, 8 - 10 April
-
Discover Exciting Career Opportunities on ISPE's Job Board
-
Steele Appointed Acting Director of US FDA's CBER
-
US FDA to Cut Back on Routine Inspections
-
Tariff Threats Create Uncertainty in Pharma Market
-
Expecting the Unexpected Ensures Continuity in Biopharma Manufacturing
-
Digital Twin may Streamline Gene Therapy Production
-
AMT Designation Granted to Cellares' Cell Shuttle
-
Innovative Culture Media Support Intensified Processes
- Navigating Category 3 Compounding Challenges