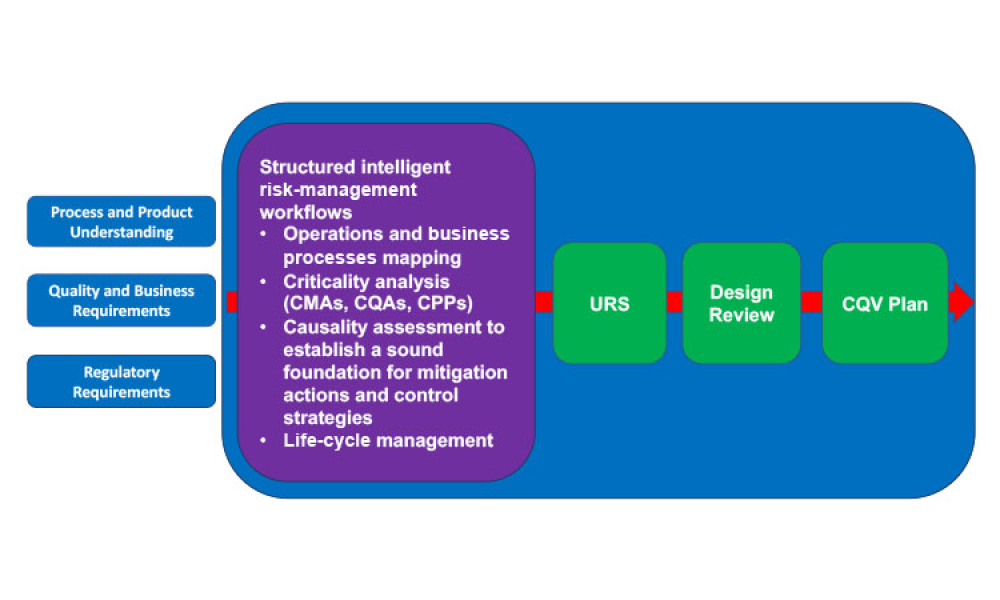
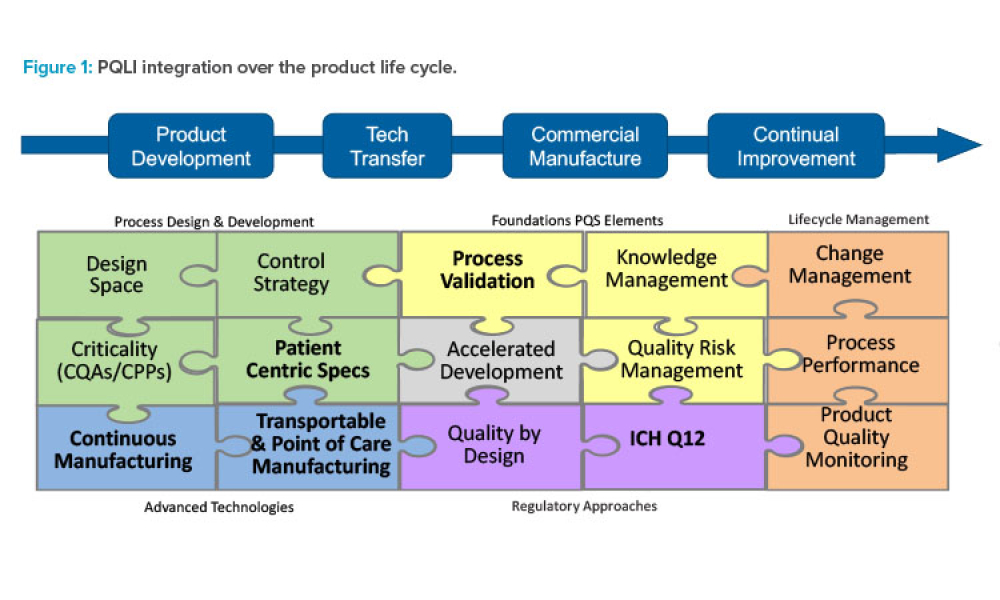
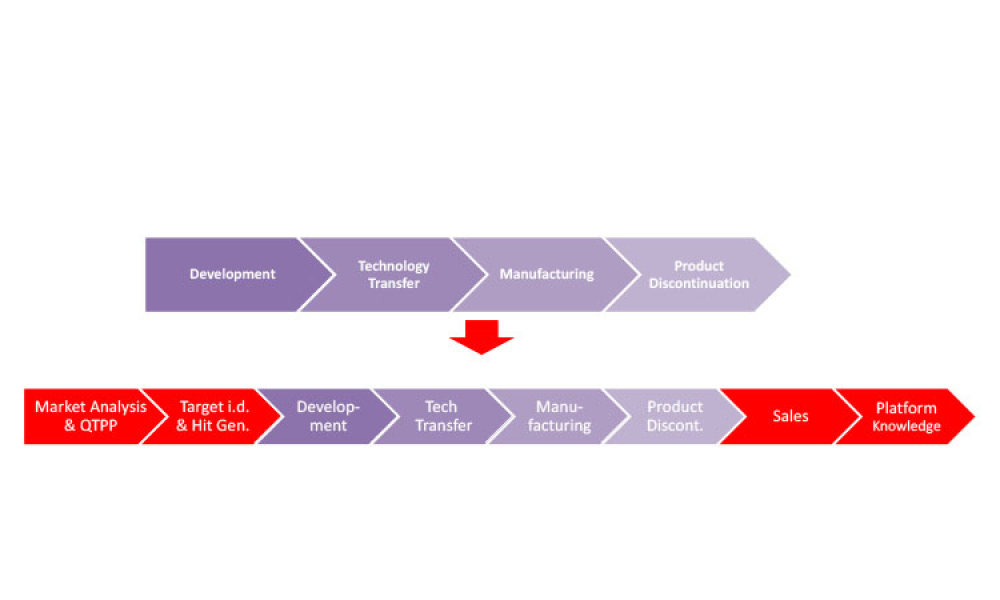
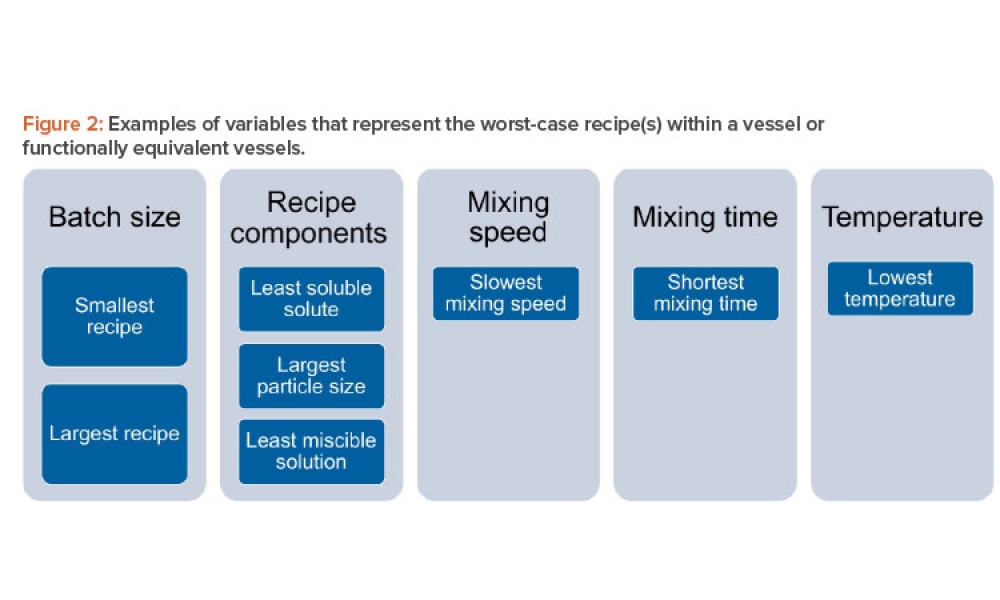
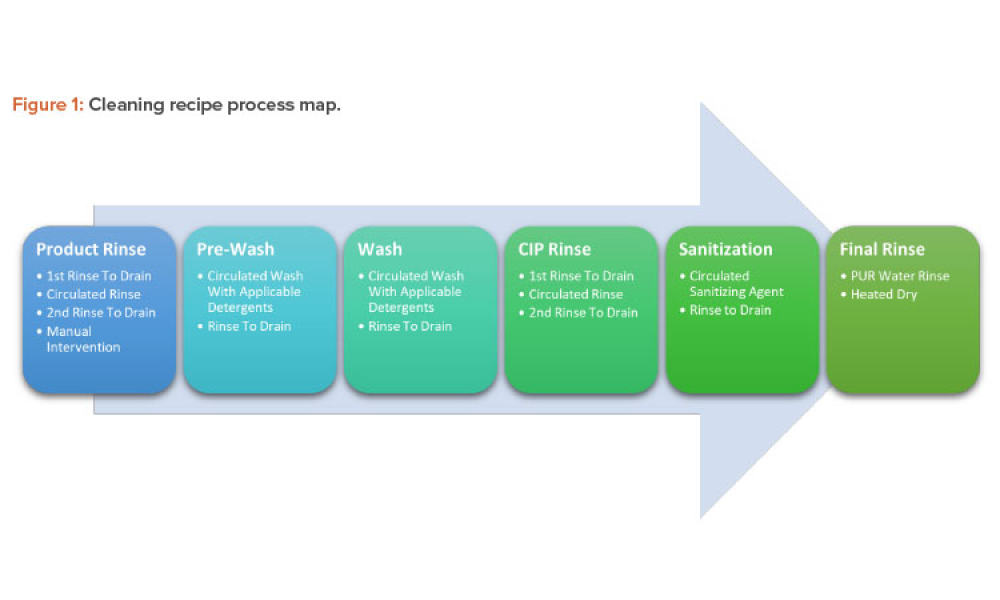
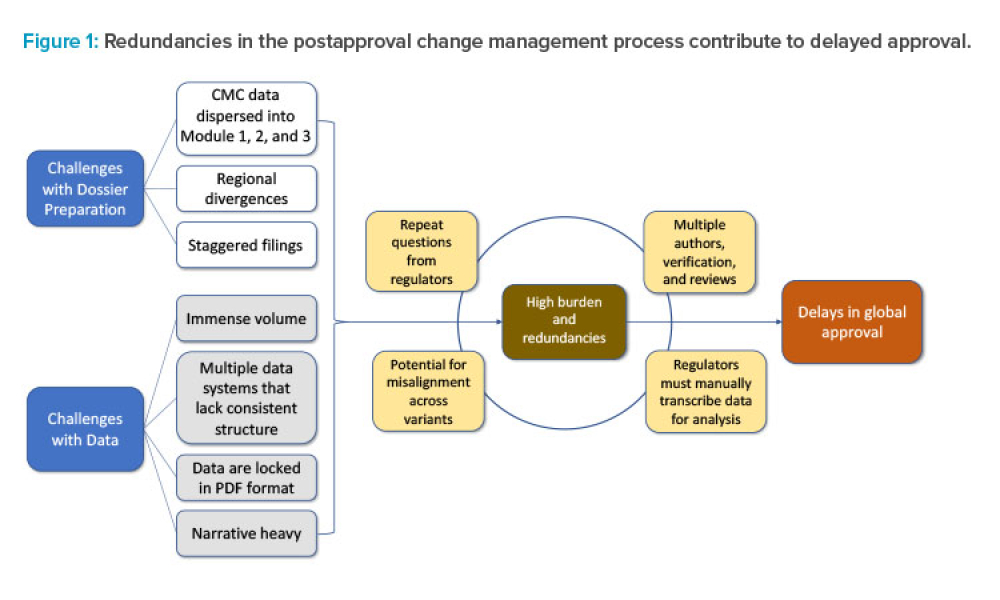
Pharmaceutical Product Lifecycle Management is the process of managing the entire lifecycle of a product from its conception, through design and manufacture, to service and disposal. Lifecycle Management forms the product information backbone for a company and its extended enterprise.
Guidance Documents
Advanced Manufacturing (1)
+Biotechnology (2)
+GAMP® (1)
+Knowledge Management (1)
+Lifecycle Management (6)
+Manufacturing Operations (1)
+Process Analytical Technology (3)
+Quality by Design (2)
+Validation (1)
+Community Discussions
Community Discussions
Apr 08, 2025
Validation
Apr 08, 2025
Data Integrity
Apr 07, 2025
Advanced Manufacturing
Biotechnology
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Mar 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Mar 20, 2025
Mar 20, 2025
Pharmaceutical Engineering Magazine Articles
Webinars
Upcoming
On-Demand
White Papers
Lifecycle Approach to Biotech Process Validation
This discussion paper proposes ideas for answering the questions about the application of the…
Stage 3 Process Validation: Applying Continued Process Verification Expectations
This discussion paper proposes ideas for answering the questions “How is Stage 3 monitoring and…