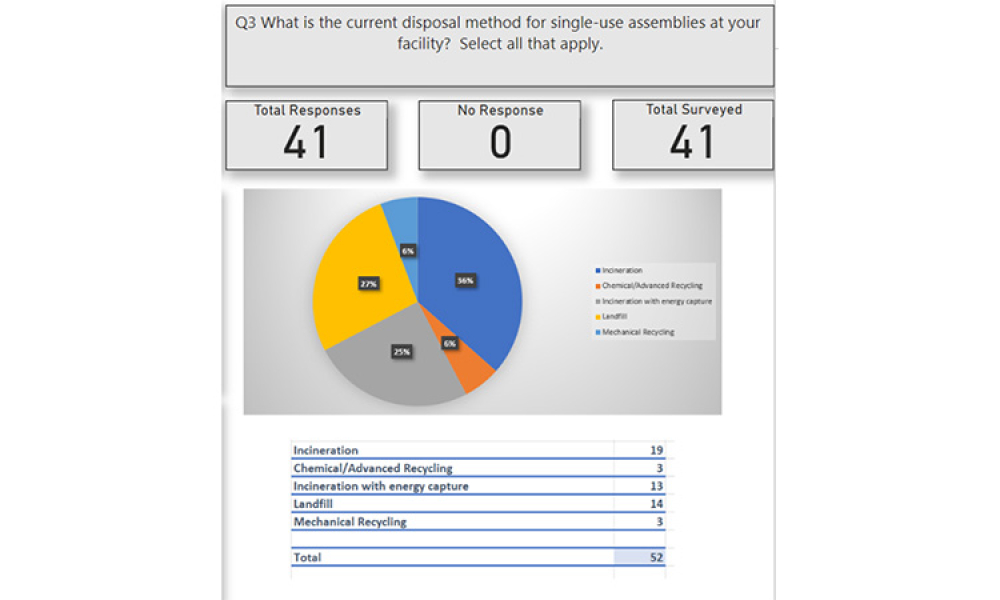
Single Use Technologies & Disposables refer to biopharmaceutical manufacturing (bioprocessing) equipment designed to be used once (or for a single manufacturing campaign) and then discarded.
Guidance Documents
Single Use Technologies & Disposables (1)
+Supply Chain Management (1)
+Community Discussions
Community Discussions
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Pharmaceutical Engineering Magazine Articles
iSpeak Blog Posts
Professional Development Training
ICH Q9(R1): Guidelines on Pharmaceutical Risk Management
+ICH Q6A: Specifications, Test Procedures, and Acceptance Criteria for New Drug Substances and Products
+GxPs for Leadership
+GMP Refresher
+Advancing Pharmaceutical Quality (APQ) Quality Management Maturity Training Course
+White Papers
July / August 2024
The explosive growth of advanced therapy medicinal products (ATMPs), particularly cellular…
May / June 2024
The commercialization of personalized medicine has ushered in demand for a new type of facility—…
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…