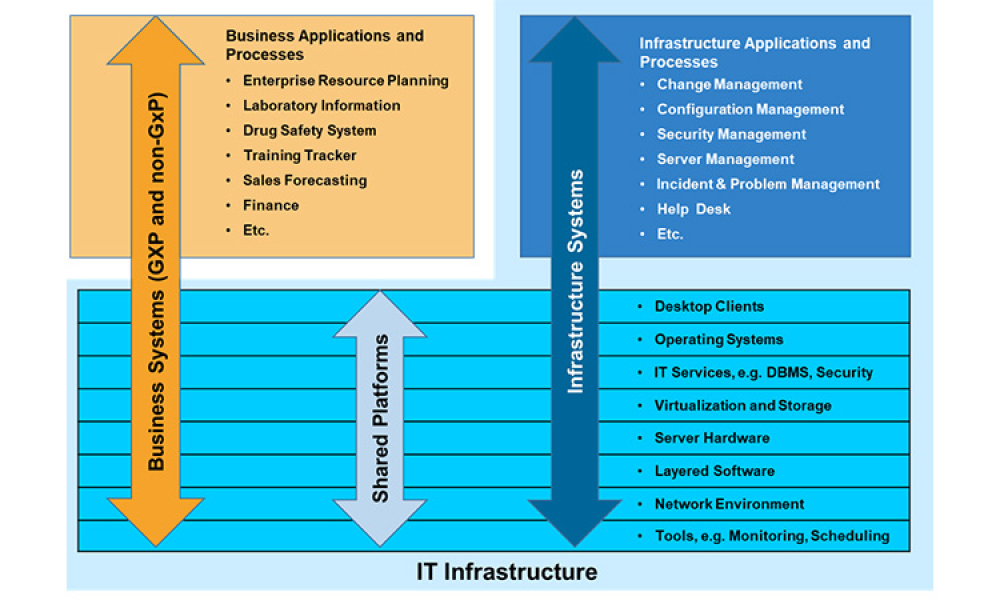
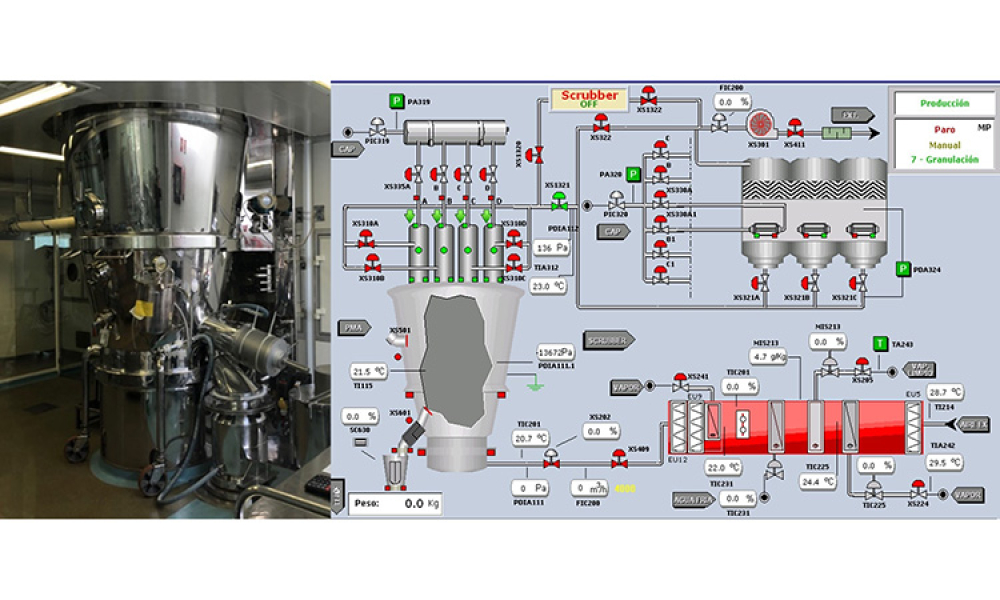
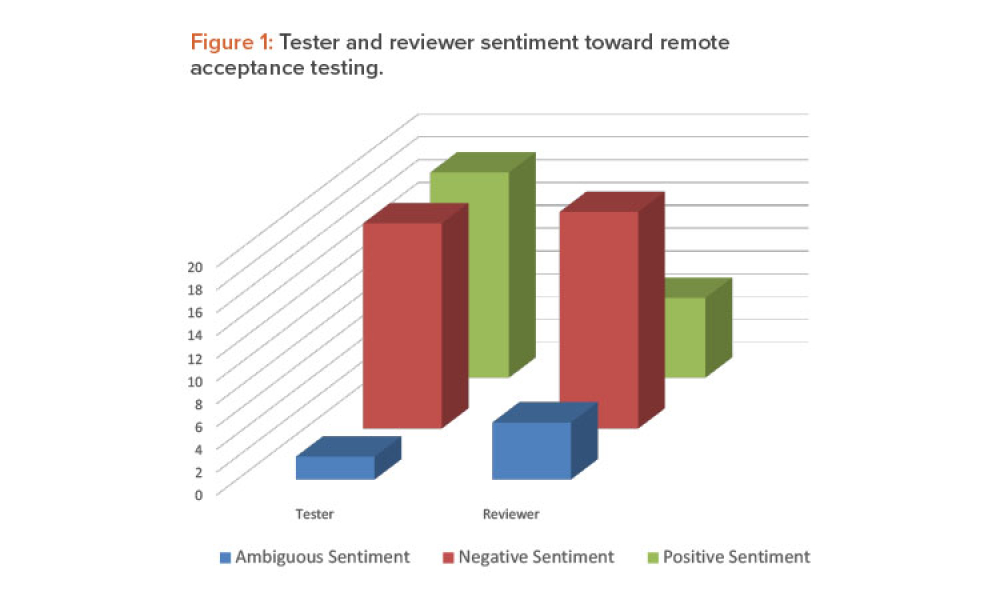
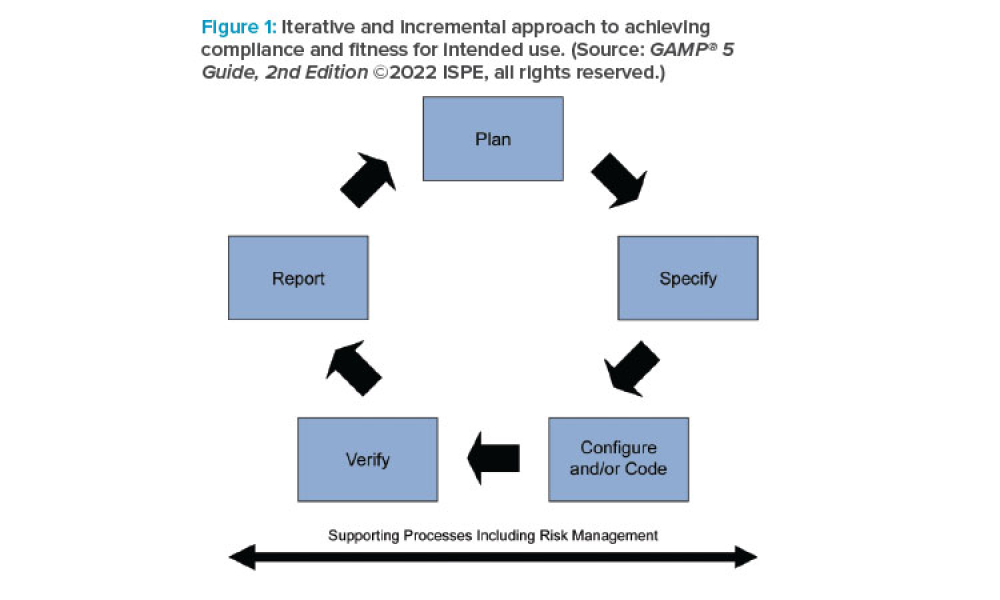
GAMP® guidance aims to safeguard patient safety, product quality, and data integrity in the use of GxP computerized systems. It aims to achieve computerized systems that are fit for intended use and meet current regulatory requirements by building upon existing industry good practice in an efficient and effective manner. GAMP® adopts a patient-centric risk-based approach that enables innovation while demonstrating compliance with regulatory requirements. GAMP® is an ISPE Community of Practice (CoP). GAMP® provides practical guidance that:
- - Facilitates the interpretation of regulatory requirements
- - Establishes a common language and terminology
- - Promotes a system life cycle approach based on good practice
- - Clarifies roles and responsibilities
The ISPE GAMP® 5 Guide: A Risk-Based Approach to Compliant GxP Computerized Systems Second Edition aims to protect patient safety, product quality, and data integrity by facilitating and encouraging the achievement of computerized systems that are effective, reliable, and of high quality. Technological innovation is essential for life sciences industries in providing value to society while also controlling costs and reducing time to market. The Guide facilitates the effective and efficient use of valuable resources by the application of appropriate and proportionate practices, encouraging innovative approaches to managing risk to patient safety, product quality, and data integrity, while supporting benefit to public health.
View Other Resources
GAMP® Guidance Documents
Good Automated Manufacturing Practice (GAMP®), is a technical sub-committee of the International Society for Pharmaceutical Engineering (ISPE). The goal of this committee is to promote the understanding of the regulation and use of automated systems within the pharmaceutical industry. The GAMP committee organizes training guides for its members. These guidelines are:
Commissioning & Qualification (1)
+Data Integrity (15)
+GAMP® (16)
+Knowledge Management (1)
+Lifecycle Management (1)
+Manufacturing Operations (1)
+Regulatory (1)
+Validation (12)
+Community Discussions
Community Discussions
Webinars
iSpeak Blog Posts
Pharmaceutical Engineering Magazine Articles
Videos
Professional Development Training
GAMP® Data Integrity 21 CFR Part 11, 2-Day Training Course
+Requirements for Computerized Systems Validation and Compliance
+Cloud Concepts - Cyber Security and Block Chain
+Basic Principles of Computerized Systems Compliance
+GAMP® Basic Principles 2-Day Training Course
+GAMP® Basic Principles 3-Day Training Course
+GAMP® 5 GxP Process Control Training Course
+GAMP® Data Integrity 21 CFR Part 11, 3-Day Training Course
+Pharmaceutical Job Board
White Papers

Featured Conferences