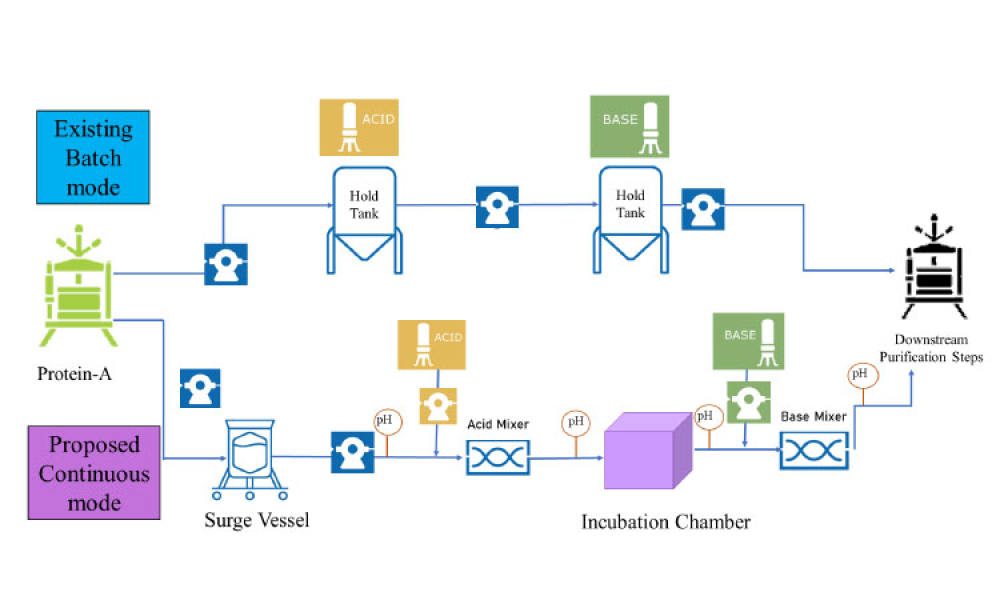
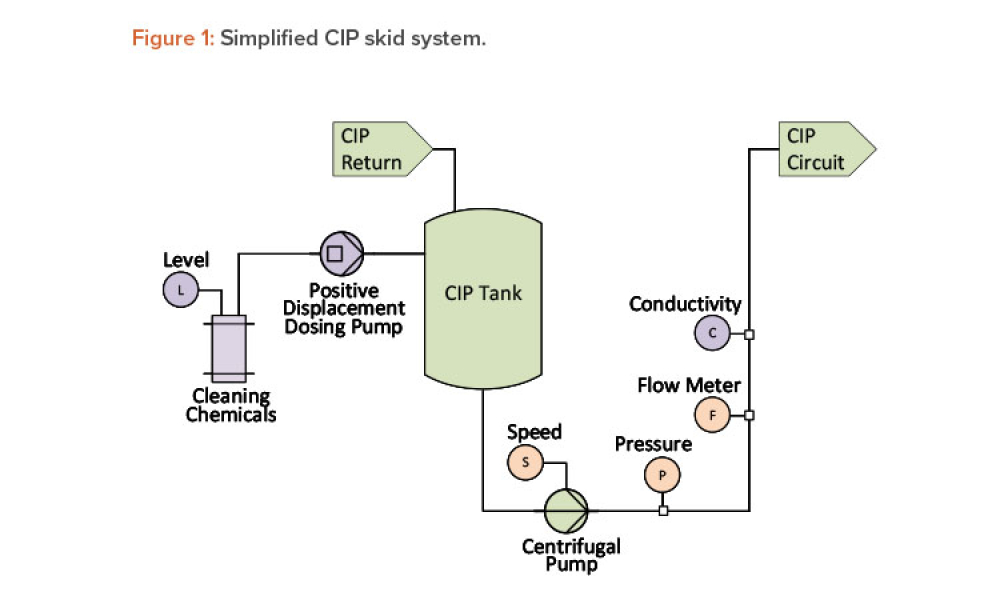
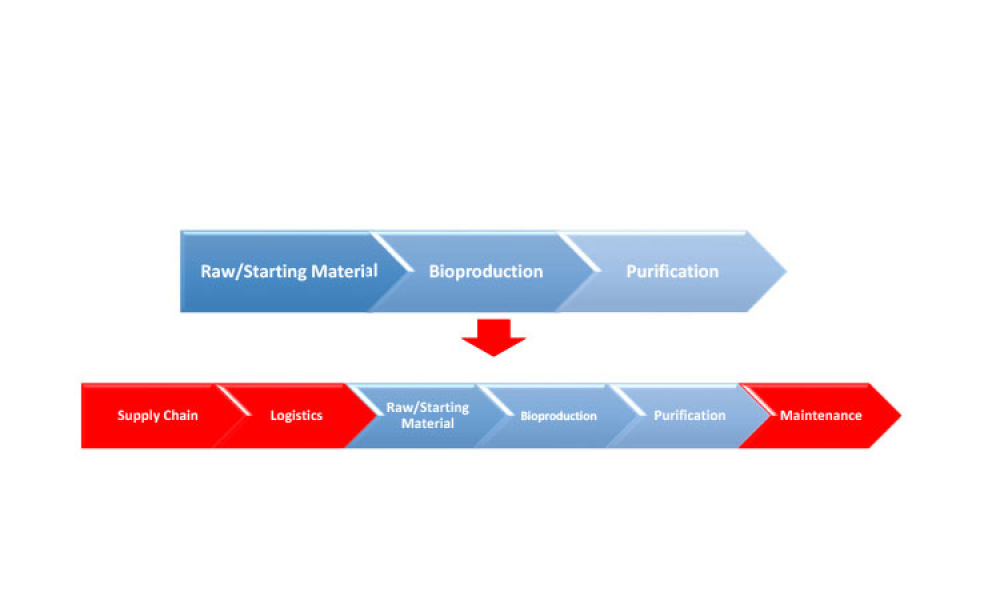
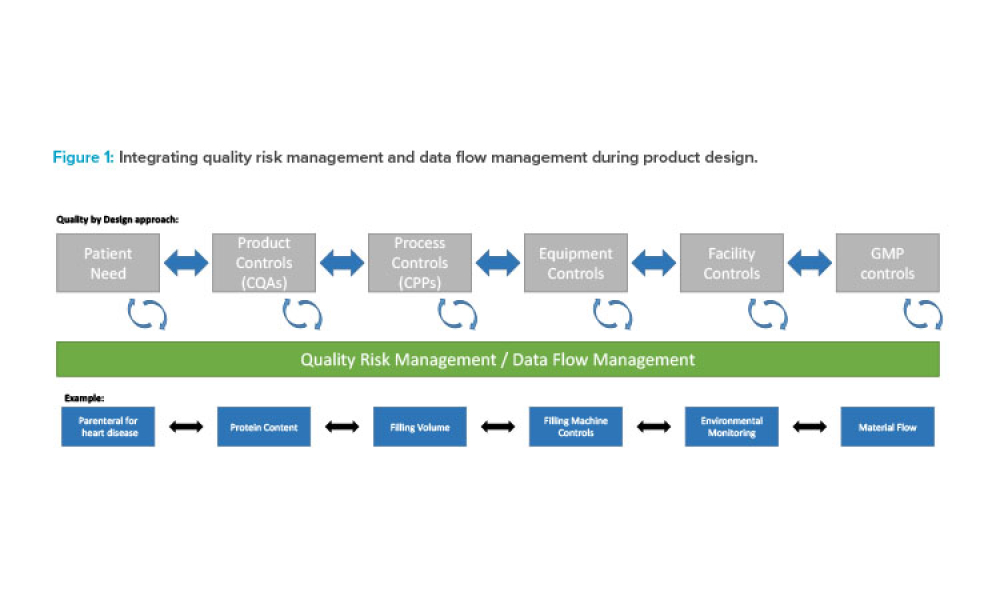
Cell and gene therapy (C>) products address various diseases at the cellular or genetic level, offer innovative treatment approaches, and represent a significant advancement in the field of medicine. However, developers of C> products face unique challenges due to their complexity, such as establishing assays that show a clear link between potency, mechanism of action (MoA), and...
Drug delivery devices have become an essential component for many modern medical therapies, and it’s vital that they function as intended. However, the reality of marketed products shows that this is not always achieved because drug-device combination products are becoming increasingly complex, with an increasing number of potential failure modes. Significant challenges for engineers include...