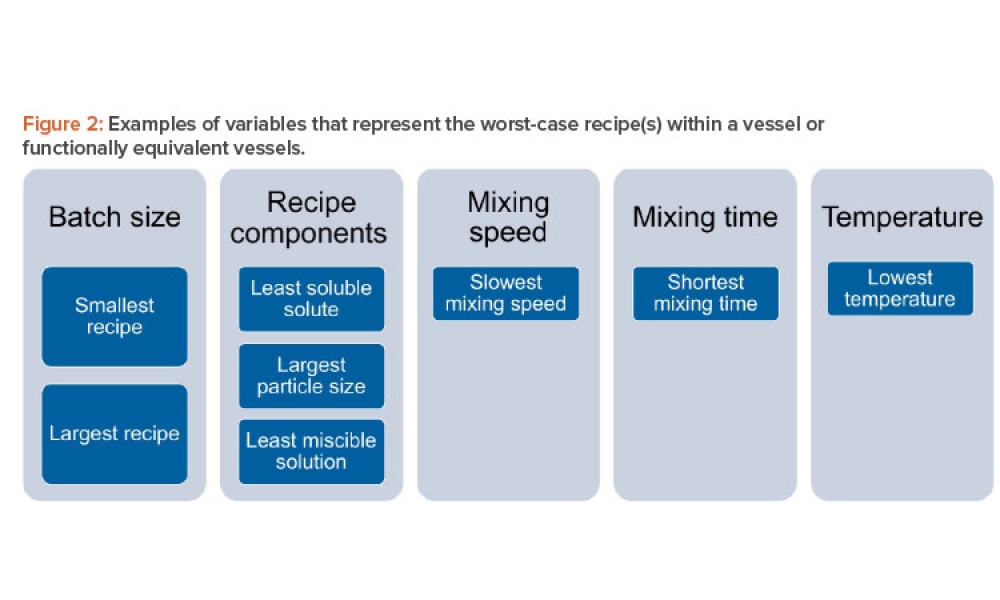
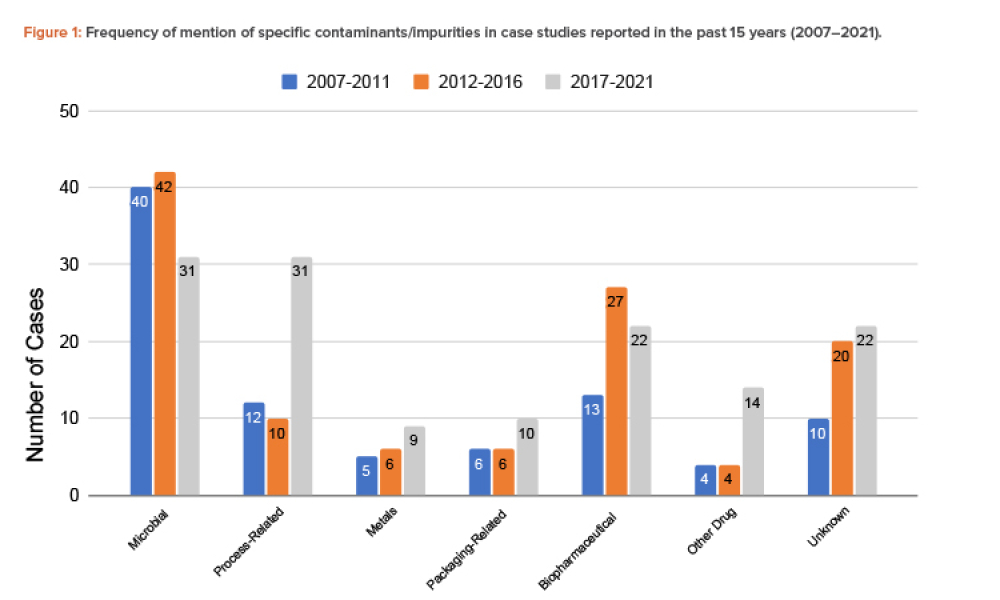
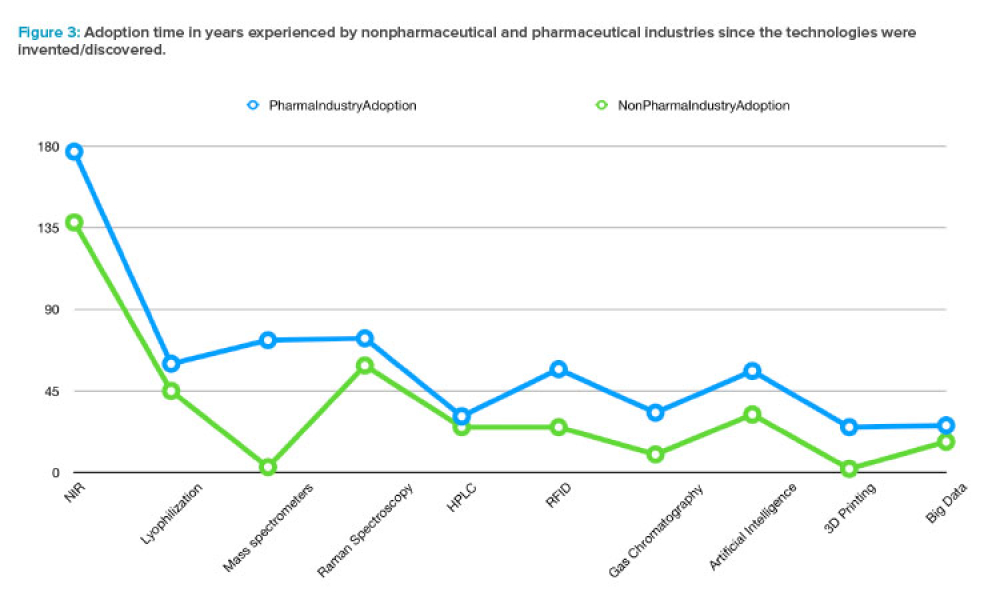
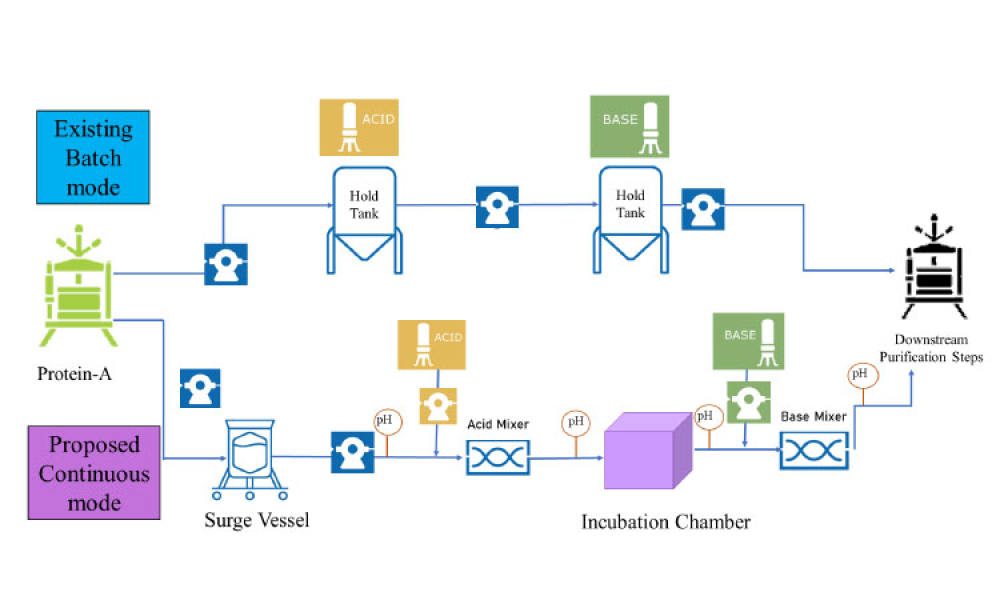
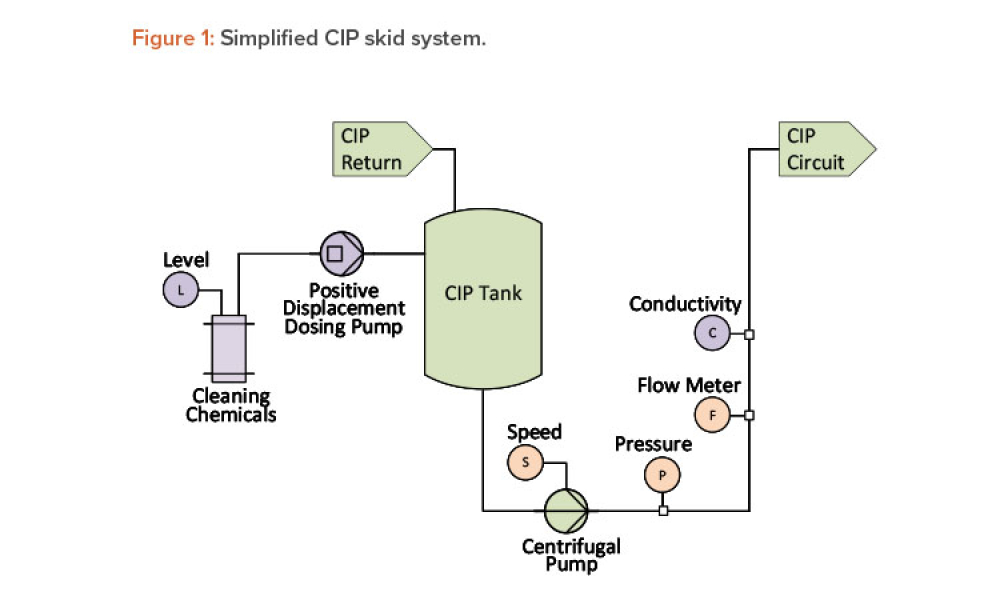
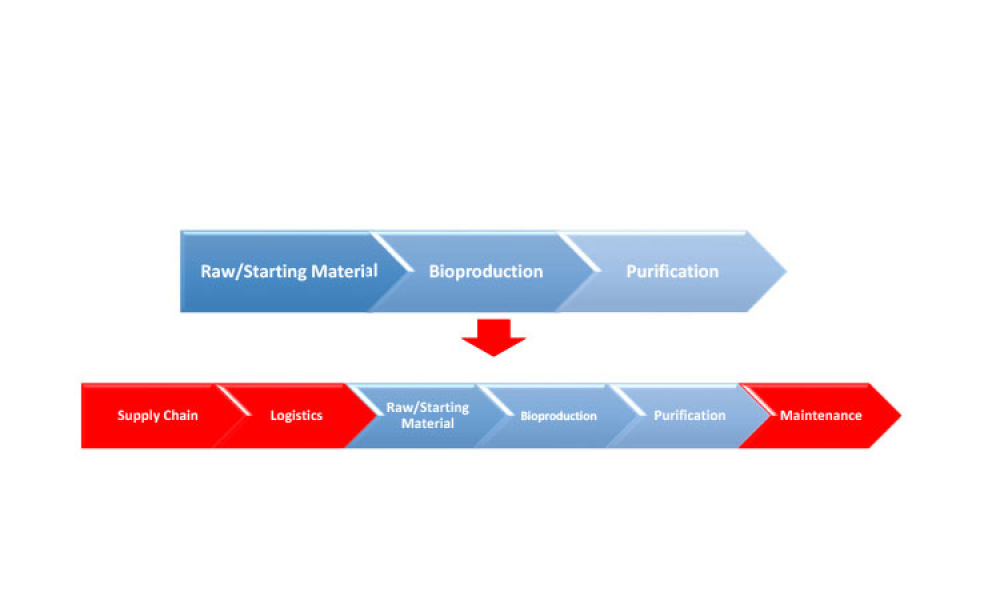
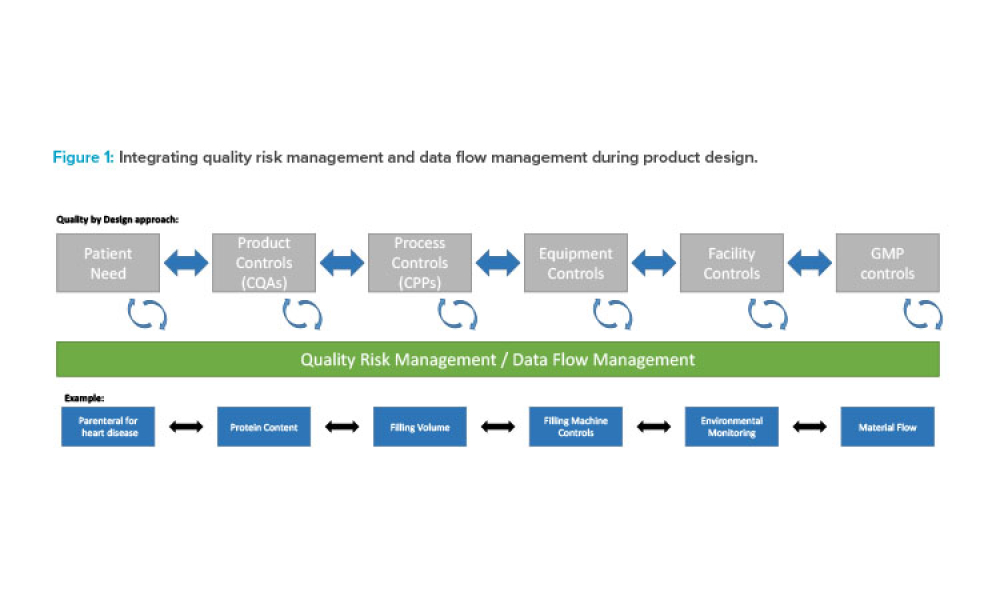
The validation of media and buffer mixing is a continuing area of resource constraint in the pharmaceutical industry. These validations require materials, validation associates’ time, and the use of equipment and processing areas. This article proposes a risk-based life cycle for minimizing mixing validation resource inputs, with the objective of optimizing validation efforts through the use...
Live biotherapeutic products (LBPs) have the potential to treat a wide range of ailments. However, these living microorganisms are difficult to produce due to evolving government regulations and limited GMP manufacturing experience. New facility designs and more specific process guidance could help overcome these challenges. This article explores the nuances of facility design and regulatory...