Current State of Oligonucleotide Therapeutics

Oligonucleotides are a relatively new class of drugs, composed of natural and synthetic nucleotides, which primarily include small interfering RNA (siRNA), micro RNA (miRNA), and antisense oligonucleotide (ASO). These molecules achieve therapeutic effects through RNA interference, degradation, or splice-modulating pathways.1, 2, 3, 4 Other oligonucleotide therapies include messenger RNA (mRNA, single strand, >500 mers),5 small activation RNA (double strand, ~20 mers),6 antagomir (single strand, ~20 mers),7 and aptamer (single strand, >30 mers).8 This article focuses on synthetic ASOs (single strand, 16–22 mers) and siRNAs (double strands formed by hybridization of a pair of complementary sense and antisense strands, 19–25 mers).
Several ASO drugs have been approved (Table 1),9, 10 including SPINRAZA (nusinersen), which has become the standard care for spinal muscular atrophy, a rare genetic disease. 11 A customized ASO drug, milasen, has also been developed to treat the genetic disorder Batten disease, which is unique to a single pediatric patient12 The therapeutic potential of siRNAs via gene-silencing mechanisms was proven for the first time by approval of the lipid nanoparticle-delivered siRNA drug ONPATTRO (patisiran) in 2018 for the treatment of the rare disease hATTR amyloidosis. GIVLAARI (givosiran; approved in 2019), a siRNA conjugate of a liver-targeting delivery system N-acetylgalactosamine (GalNAc), further opened opportunities for siRNAs to treat liver-expressed diseases. For example, the GalNAc-conjugate drug inclisiran, which targets PCSK9, shows great promise as a transformational medicine for atherosclerotic heart disease and familial hypercholesterolemia in chronic cardiovascular disease patients at higher risk of cardiovascular events due to high low-density lipoprotein cholesterol levels.13
| Drug | Year Approved |
|---|---|
| Fomivirsen | 1998 (US FDA) and 1999 (EMA) |
| Mipomersen | 2013 (US FDA) |
| Eteplirsen | 2016 (US FDA) |
| Nusinersen | 2016 (US FDA) and 2017 (EMA) |
| Inotersen | 2018 (US FDA and EMA) |
| Patisiran | 2018 (US FDA and EMA) |
| Volanesorsen | 2019 (EMA) |
| Givosiran | 2019 (US FDA) |
| Golodirsen | 2019 (US FDA) |
| Viltolarsen | 2020 (US FDA) |
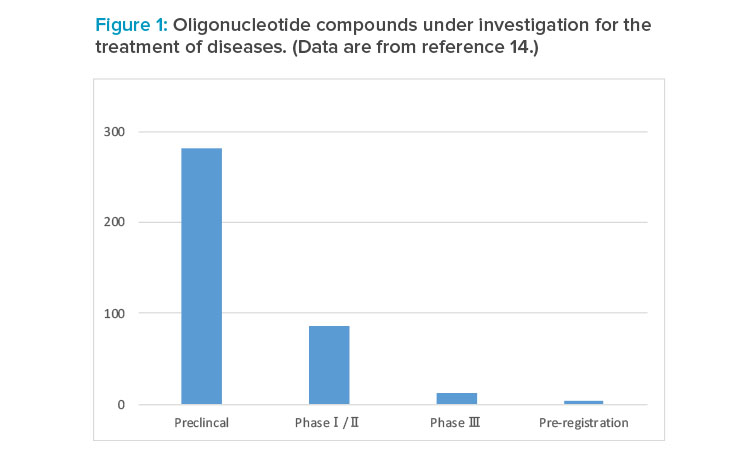
Strong interest in oligonucleotide therapy is evidenced by the 386 compounds being investigated in various research and development stages (Figure 1),14of which 76% are chemically synthesized oligonucleotides. They cover rare and common infectious (e.g., chronic hepatitis B), cardiometabolic, and central nervous system diseases.
The clinical development of these complex molecules poses challenges and opportunities from a chemistry, manufacturing, and controls (CMC) perspective. The drug substance manufacturing operations that present the most difficult challenges involve the chemistry, process, and impurity characteristics.
Oligonucleotide Synthesis Technologies
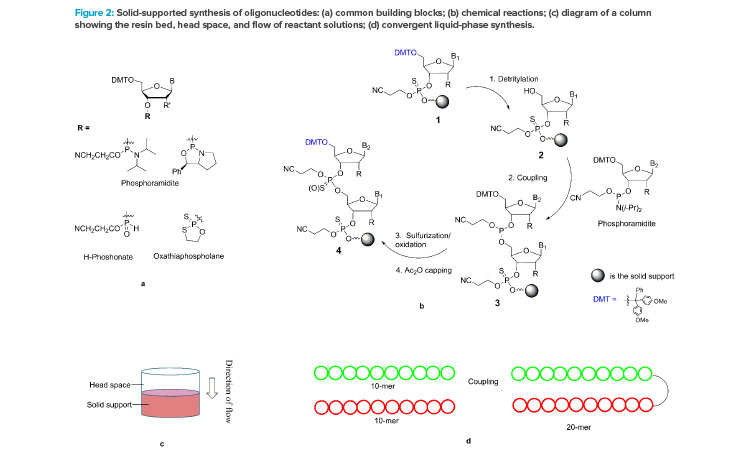
The chemical synthesis of oligonucleotides is based on the iterative coupling reaction of nucleotide starting materials (SMs), which contain both a 5’-DMT protecting group and a 3’-reactive phosphorous linking group. The phosphorous linking group is typically a phosphoramidite, H-phosphonate, or oxathia-phospholane (Figure 2a).15, 16,17 The phosphoramidite is most widely used in the synthesis from nanomole to molar scales. Both phosphoramidites16 and oxathiaphospholane18 have been developed for the synthesis of stereochemically pure phosphothioate linkages.
A repeating synthesis cycle is employed to build an oligonucleotide chain by chemically connecting the nucleotide starting materials, one at a time, to a solid support, such as resin (see steps 1–4 in Figure 2b). Operationally, each reaction is carried out by pumping a solution of the starting material or reagent through a column packed with the solid support (Figure 2c) and then washing the resin-bound product with a suitable solvent. After the four-reaction cycle is completed, the single nucleotide is added, and the growing chain (step 4) has the same 5’-DMT functional group as step 1. Therefore, the same four-reaction cycle can be repeated to add the next nucleotide, until the desired chain is assembled. Note that step 4, capping with the acetic anhydride, is used to control deletion impurities (n-1) by converting the unreacted 5’-OH to acetate.
This elegant synthesis has been automated. A synthesizer equipped with a computer can carry out an oligonucleotide synthesis without human intervention. A parallel synthesizer can produce hundreds of oligonucleotides each at milligram quantity in as little as 3 hours, and the synthesis of a single oligonucleotide up to 7 kg per batch can be accomplished in less than 24 hours. Parallel synthesis of long-chain oligonucleotides (150 mers) is also possible.19
The synthesis reactions occur when a solution of a starting material or a reagent flows through the column. The reaction times (i.e., the duration of the solution deliveries through the column) and volumes of the reactant solutions can both be controlled precisely by the computerized synthesizers. Though it is true that the dynamic contact of solutions with the resins can vary with change in column size, particularly with swellable resins, extraordinarily similar results can be obtained from syntheses carried out at a broad range of scales (e.g., from 2 to 600 mmol), when the reaction conditions and the resin bed heights are kept the same. This phenomenon indicates that possible changes in mass transfer in the range of scales has no impact on reaction efficiency, despite more than 10-fold differences in column diameters and the constant swelling changes of the resin bed throughout the synthesis.
For solid-supported synthesis to work, the reactions must be high yielding with little side product generation, and the starting materials must limit the levels of impurities that are reactive and can be incorporated into the growing chain. This is because the reaction cycle is repeated many times and impurities are generated on the growing chain in each cycle. Similarly, an impurity in a starting material that can be incorporated into the growing chain generates one impurity every time the starting material is used. Most of these impurities cannot be removed from the desired full-length product in the purification process. Therefore, the small quantities of impurities generated in each reaction and from starting material add up to a high level of total impurities. For example, one impurity at 1% incorporation into a 20-mer product in each cycle would result in about 20% impurity. For the same reason, a small change in quantities of process and starting material impurities can result in a large change in total impurities. Fortunately, most of the oligonucleotide therapy platforms are based on starting materials with chemically stable substituents on the sugar ring20 and well-established supply chains. The synthesis process is well optimized, and most of the pro-cess-related impurities are adequately controlled.
Oligonucleotides can contain many structurally related impurities that cannot, at present, be separated and quantified individually. In practice, the oligonucleotide impurities are grouped and quantified as groups, mostly based on their structure characteristic.21 For example, deletion impurities such as n-1 are a group of impurities in which one of the nucleotides is missing from the full-length oligonucleotide. This deletion can happen at any position in the sequence where a repeated individual nucleotide is supposed to be present. Despite the current limitations of specificity, highly sensitive analytical technologies such as high-performance liquid chromatography with ultraviolet and mass spectroscopy (HPLC-UV-MS) methods are indispensable for developing a manufacturing process and establishing a control strategy to ensure acceptable product purity across each stage of clinical development.
Large-Scale Synthesis
Solid-supported synthesis will remain the mainstream production method for oligonucleotide drug discovery, early-stage clinical studies, and even late-stage clinical investigations and commercialization to treat rare diseases. However, with the anticipated success of oligonucleotide drugs to treat diseases affecting large patient populations [3], a cost-effective and sustainable large-scale synthesis process is needed. For example, over a metric ton of a single drug may be needed per year to treat Alzheimer’s disease or cardiovascular disease. To meet such a demand, around 200 batches of the syn-thesis are needed at the current maximal scale of approximately 1 mole and approximate 55% yield. To gain economies of scale, it is conceivable that the synthesis unit operations may be scaled up further, on the basis of the remarkably similar results obtained from 2- to 600-mmol scales, despite the technical challenges foreseen with even larger columns and flow rates topping hundreds of liters per minute.22 Furthermore, the cost to build synthesizers and related facilities is high. Clearly further scale-up is a formidable undertaking. Alternatively, increasing the yield—for example, to 90% from the current approximate yield of 55%—could substantially reduce the cost by eliminating roughly 90 of the 200 runs. This is not impossible, considering the fact that about 75% yield was obtained by removing the capping step.23
Large-scale solution-phase manufacturing of small molecule pharmaceuticals has existed for decades, and spare capacity is available around the world. Consequently, processes suitable for these facilities have considerable advantages financially and environmentally.
Investigators have extensively explored synthesis that uses soluble instead of solid anchors and can be performed without synthesizers and columns, although only synthesis of a morpholino oligonucleotide has been demonstrated at the 10-kg-per-batch scale.24 However, it is not far-fetched to expect that this approach can be scaled up further. More recently, proof-of-concept studies focusing on separation of intermediates using membrane filtration at laboratory scale have been reported.25 Synthesis using template-dependent26 or independent 27 enzymatic reactions has been investigated. Nevertheless, many technical obstacles must be overcome, particularly for oligonucleotides containing unnatural nucleotides and phosphorothioate linkages.20
Most issues in the linear synthesis employed in solution-phase synthesis could be alleviated by developing convergent processes. Figure 2d illustrates a convergent synthesis of a 20-mer from two 10-mers. Such an approach has been used in peptide manufacturing.28 This strategy minimizes impurity generation and yield loss by reducing unnecessary exposure and burden to carry the growing oligonucleotide through many reactions and isolation steps, and it reduces the impact of failure because the synthesis of two 10-mers is independent. The few studies on convergent synthesis reported include the synthesis of a 6-mer by a 3 + 3,29 trimers for gene libraries,30, 31 and ASOs using a template-based ligation of fragments.26 A promising convergent template-free synthesis of double-strand siRNAs from short oligonucleotide fragments using enzyme catalysis was disclosed at the September 2020 US TIDES: Oligonucleotides and Peptide Therapeutics Conference. We believe convergent chemical synthesis can provide cost-effective and sustainable manufacturing processes in the near future.
Conclusion
Solid-supported synthesis fulfills the need for early-stage development and commercialization of drugs for rare diseases. Multiple production lines may be built to produce hundreds of kilograms of drugs to treat common diseases. However, we foresee that successful clinical development of drugs to treat diseases that afflict millions of patients will create demands for metric tons of drugs. In our opinion, such demands can be met only by developing large-scale solution-phase convergent synthesis due to its intrinsic advantages for multistep synthesis, such as in the synthesis of a typical oligonucleotide, which requires at least 54 reactions to prepare.
About the Authors
Acknowledgements
The authors thank William F. Kiesman, PhD, and Firoz D. Antia, PhD, of Biogen for their feedback in the preparation of this article.



