Continuous manufacturing (CM) challenged regulators’ expectations and regulatory frameworks. This article discusses how US regulators addressed the regulatory hurdles related to CM to broaden its adoption through engagement, regulatory science, guidance, and international harmonization.

Downloads
Removing Regulatory Hurdles for Continuous Pharmaceutical Manufacturing
Cover: Continuous manufacturing (CM) challenged regulators’ expectations and regulatory frameworks. This article discusses how US regulators addressed the regulatory hurdles related to CM to broaden its adoption through engagement, regulatory science, guidance, and international harmonization.
ICH Q13 and What Is Next for Continuous Manufacturing
Feature: The creation of a new ICH guidance document, Q13 [1], presents an opportunity for industry and regulators around the world to connect and develop harmonized regulatory expectations for the continuous manufacturing (CM) of drug substances and drug products, resulting in an increased likelihood of implementation across the globe.
Agile, Data-Driven Life Cycle Management for Continuous Manufacturing
Feature: Pharmaceutical continuous manufacturing (CM) is recognized as a key process intensification technology, with investment expected to rise in the coming years and the focus shifting toward biologics. This article provides a review on the current state of CM implementation, offers insights into life cycle management and regulatory aspects, and explains how a data and knowledge-centric approach to risk management can help CM achieve its full potential.
ChatGPT, BARD, and Other Large Language Models Meet Regulated Pharma
Technical: ChatGPT and other large language models are positioned to change the world. They can also shift acceptance and prevalence of machine learning solutions in regulated industries in general. However, their arrival requires reconsiderations on risks, quality assurance, and validation from a GxP perspective.
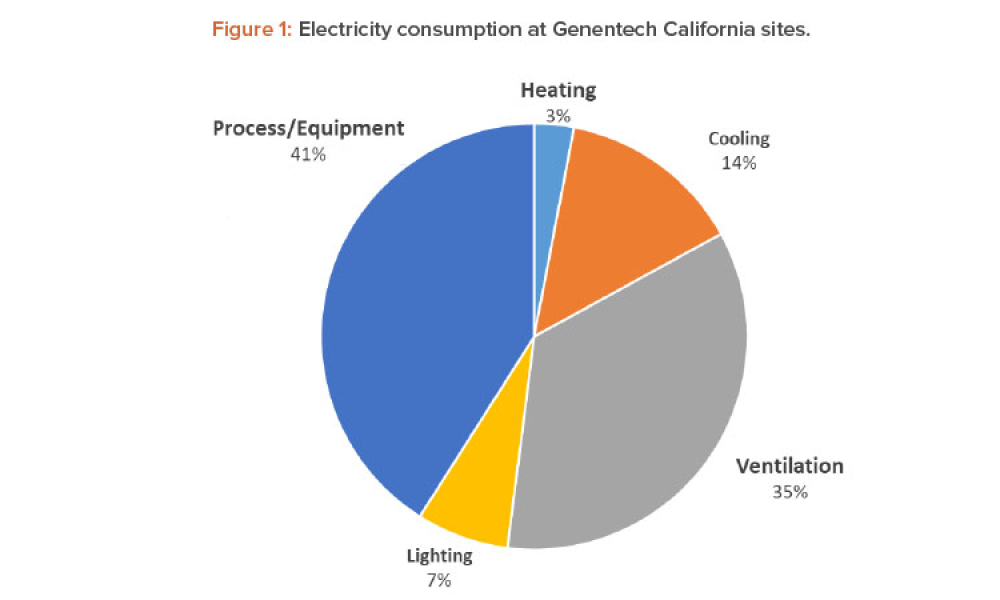
Air Change Rate Reduction during Operation: Success at Roche/Genentech
Technical: To improve the energy efficiency of cleanrooms, the Roche Global Engineering and Oceanside facilities and Engineering team collaborated to implement a risk-based approach to achieve lower air changes during operation without adversely impacting the facility, equipment, or reliability, while meeting environmental requirements.
In This Issue
It is starting to become more real that my term as Chair is winding down because the 2023–2024 International Board elections are happening.
The multigenerational environment we work in poses unique challenges that make effective communication skills as essential today as they have ever been.
The pharmaceutical industry is highly regulated, with strict standards for the production and distribution of medications. However, the concept of continuous manufacturing (CM) has revolutionized the way pharmaceutical products are made.
In the interest of understanding the current state of continuous manufacturing for biologics and to facilitate the path toward adoption of these promising technologies, the United States Pharmacopeia (USP) and BioPhorum jointly sponsored a hybrid workshop. This article summarizes trends from the workshop and ponders next steps.
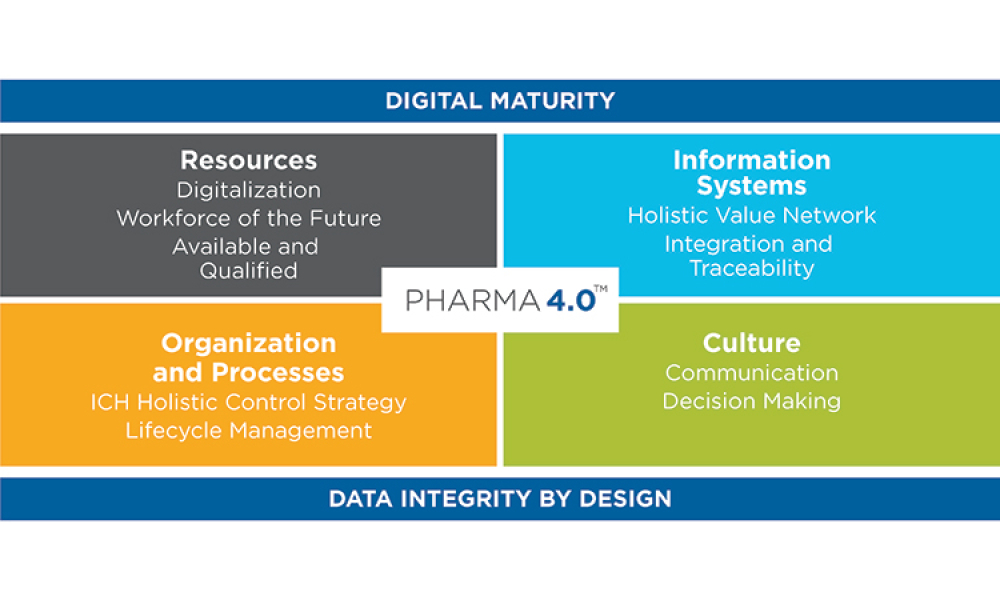
Christian Wölbeling has been a member of ISPE for 25 years. A founding member and current Chair of ISPE’s Pharma 4.0™ Community of Practice (CoP) Steering Committee, Christian has also been very involved with the GAMP® and Process Analytical Technology CoPs, as well as the ISPE Germany/Austria/Switzerland (D/A/CH) Affiliate.
The United Kingdom Emerging Leaders Awards program is an exciting initiative for ISPE because it recognizes and celebrates the efforts and contributions of young engineers in the United Kingdom pharmaceutical engineering and manufacturing sectors.
ChatGPT and other large language models are positioned to change the world. They can also shift acceptance and prevalence of machine learning solutions in regulated industries in general. However, their arrival requires reconsiderations on risks, quality assurance, and validation from a GxP perspective.
To improve the energy efficiency of cleanrooms, the Roche Global Engineering and Oceanside facilities and Engineering team collaborated to implement a risk-based approach to achieve lower air changes during operation without adversely impacting the facility, equipment, or reliability, while meeting environmental requirements.
Pharmaceutical continuous manufacturing (CM) is recognized as a key process intensification technology, with investment expected to rise in the coming years and the focus shifting toward biologics. This article provides a review on the current state of CM implementation, offers insights into life cycle management and regulatory aspects, and explains how a data- and knowledge-centric approach...
Understanding and managing risks to continuous manufacturing (CM) technology is central to any decision to greenlight CM in a production-ready environment. Applying a systemwide risk management (SRM) approach to manufacturing is essential to ensuring manufacturing projects are vetted in a comprehensive and consistent manner.
The pharmaceutical industry began applying the principles of continuous processing to the manufacture of oral solid dosage (OSD) forms in the mid-2000s. The consensus among experienced practitioners is that the continuous approach has numerous benefits. “Continuous manufacturing provides for a full range of product life cycle, from small volume clinical production to large volume commercial...
The fifth Pharma 4.0™ conference was held December 2022 in Vienna, Austria, in combination with the Aseptic Processing conference. Nearly 500 participants attended either in person or online to learn about the latest developments.
The adoption of continuous manufacturing (CM) in the pharmaceutical industry is increasing because of the advantages and opportunities it offers, including the ability to handle hazardous reactions, a higher degree of process integration, and improved sustainability. This article discusses the considerations and approaches adopted by Pfizer, Eli Lilly, GSK, and Amgen in designing and...