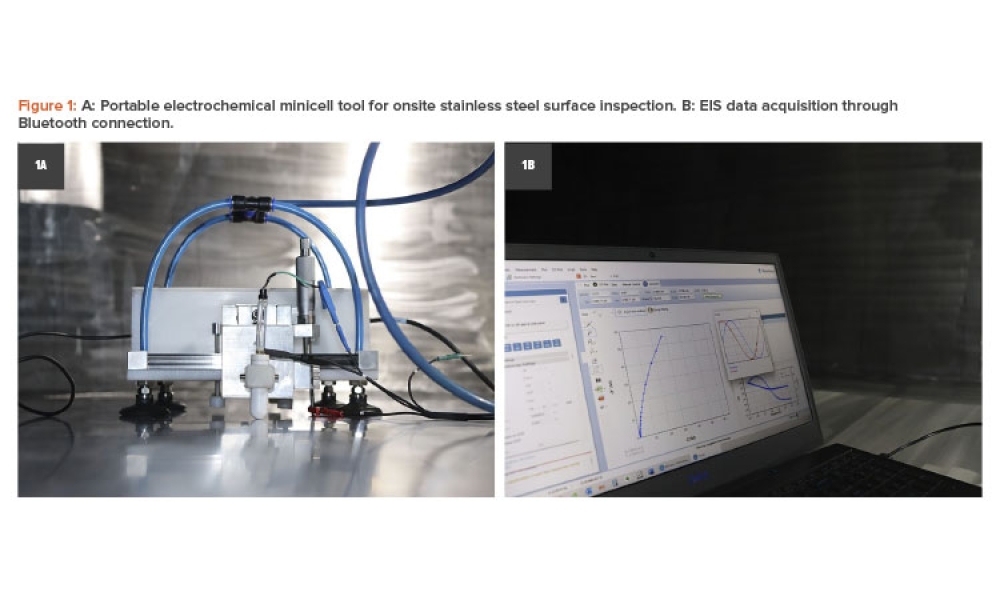
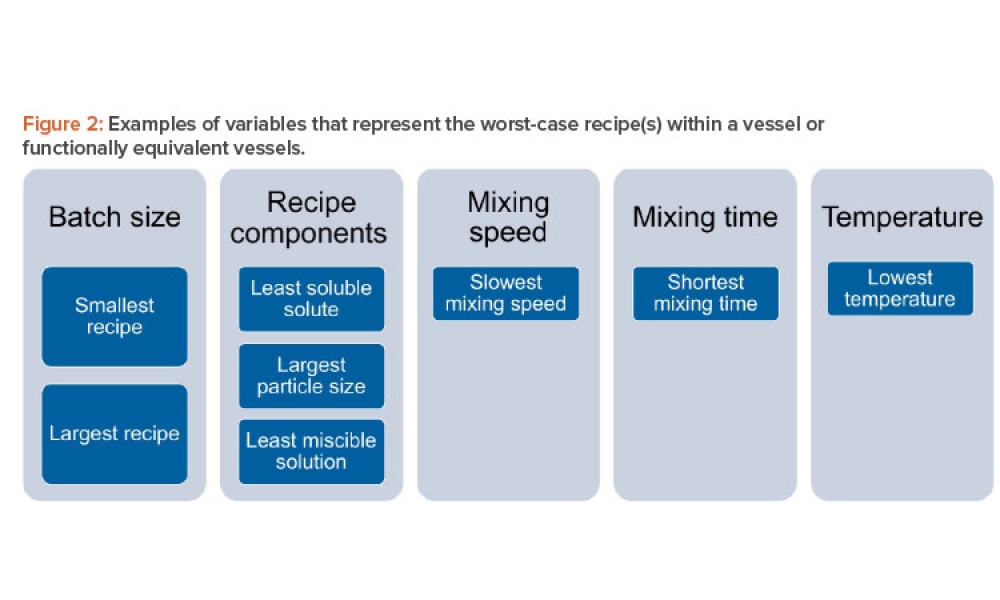
Content focuses on the role and elements of a quality management system, its impact on the overall risk management approach, and its implementation scientifically and pragmatically. Case studies could demonstrate risk management's purpose, elements, and implementation, a quality management system, and systems validation.
Guidance Documents
Biotechnology (1)
+Commissioning & Qualification (4)
+Compounding (1)
+Containment (1)
+Data Integrity (15)
+Drug Shortages (1)
+GAMP® (15)
+Good Manufacturing Practice (1)
+Knowledge Management (6)
+Lifecycle Management (3)
+Manufacturing Operations (1)
+Microbiological & Viral Contamination Control (2)
+Pharma 4.0™ (1)
+Process Analytical Technology (4)
+Project Management (1)
+Quality Assurance (8)
+Quality Control (1)
+Quality by Design (4)
+Regulatory (2)
+Sustainable Facilities, HVAC, & Controlled Environments (1)
+Validation (13)
+Community Discussions
Community Discussions
Apr 08, 2025
Validation
Apr 08, 2025
Data Integrity
Apr 07, 2025
Advanced Manufacturing
Biotechnology
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Mar 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Mar 20, 2025
Mar 20, 2025
Pharmaceutical Engineering Magazine Articles
Webinars
Upcoming
On-Demand
ISPE in the News
Latest
-
Coming Soon and Recently Published ISPE Guidance Documents
-
Validation of an Autoclave Cycle
-
Polpharma Group Invests in Manufacturing in Kazakhstan
-
USP Microbiology Lead on the Importance of Microbial Preservation
-
BDD Pharma Opens Facility near Glasgow
-
Biomanufacturers Advancing Cell Culture Techniques
-
Considerations for Analytical Testing in GMP Settings
-
Manufacturers Take Steps to Reduce SUT Waste
-
EU Pharma Industry Braces for US Tariffs
- ISPE Webinar: QRM Based Integrated C&Q Series – Good Engineering Practices (GEP) as a Key Enabler for Integrated C&Q
White Papers
May / June 2024
The commercialization of personalized medicine has ushered in demand for a new type of facility—…
January / February 2024
Stakeholders across industries are becoming accustomed to using information technology (IT) systems…
September / October 2023
Regulatory Trends & Quality Initiatives - Shortages of essential medicines around the world have…
July / August 2023
Continuous manufacturing (CM) challenged regulators’ expectations and regulatory frameworks. This…
May / June 2023
North Carolina’s Research Triangle is the largest of its kind in the US. Thanks to years of effort…