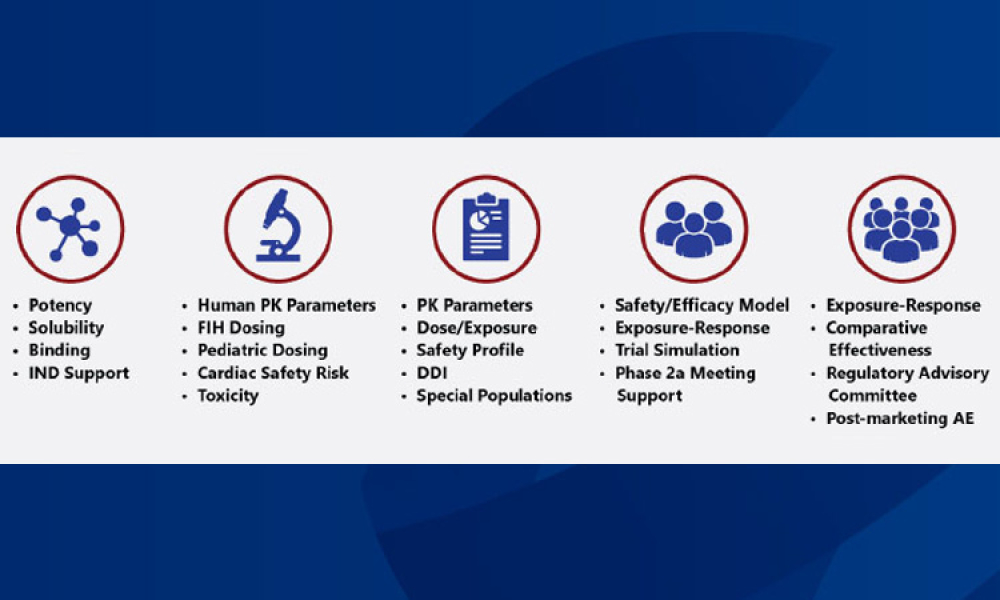
Investigational Products (Invest Prod) refers to a preventative (vaccine), a therapeutic (drug or biologic) device, diagnostic, or palliative used in a clinical trial. An investigational product may be an unlicensed or licensed product when used or assembled (formulated or packaged) differently from the approved form, when used for an unapproved indication, or when used to gain further information about an approved use.
Guidance Documents
Biotechnology (1)
+Investigational Products (8)
+Supply Chain Management (1)
+Pharmaceutical Engineering Magazine Articles
White Papers
July / August 2024
The explosive growth of advanced therapy medicinal products (ATMPs), particularly cellular…
May / June 2024
The commercialization of personalized medicine has ushered in demand for a new type of facility—…
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…

Pharmaceutical Job Board
iSpeak Blog Posts
Professional Development Training
ICH Q9(R1): Guidelines on Pharmaceutical Risk Management
+ICH Q6A: Specifications, Test Procedures, and Acceptance Criteria for New Drug Substances and Products
+GxPs for Leadership
+GMP Refresher
+Advancing Pharmaceutical Quality (APQ) Quality Management Maturity Training Course
+Featured Conferences