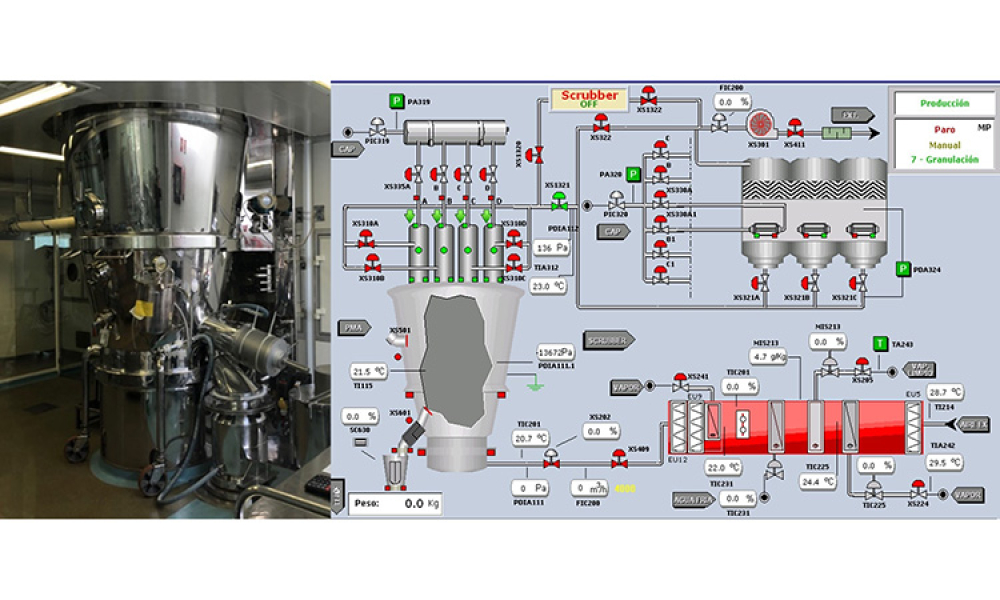
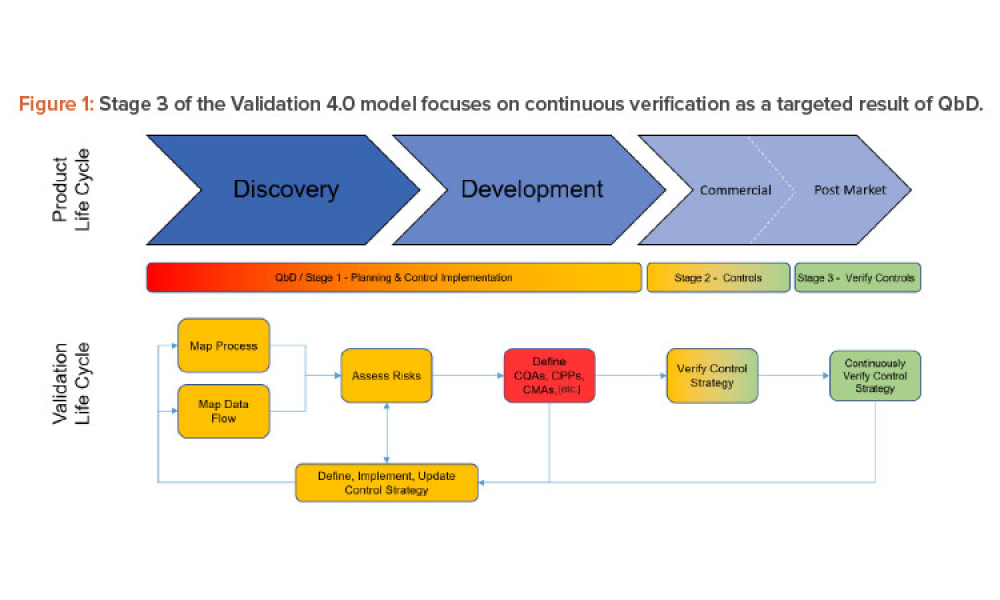
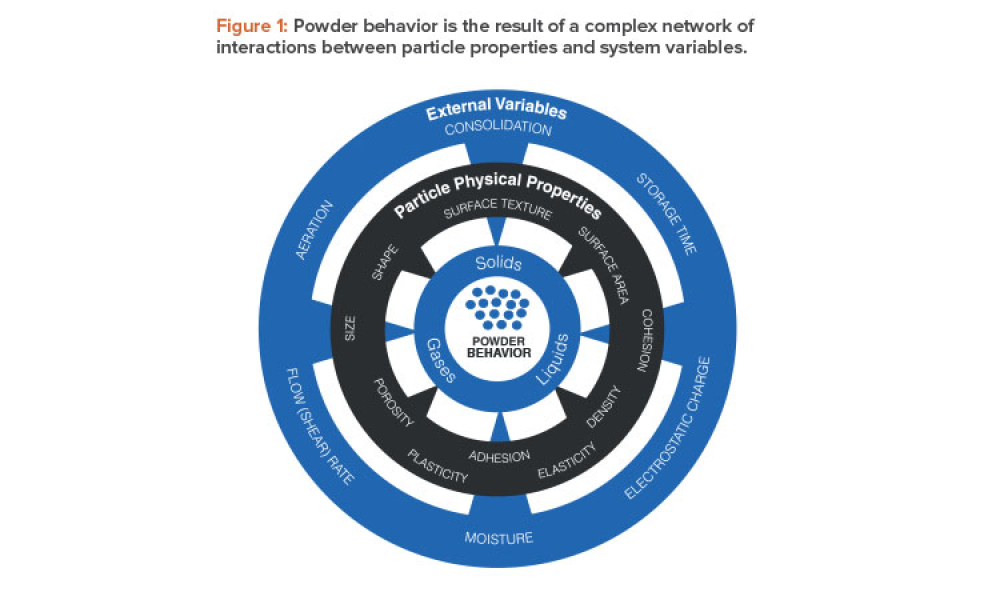
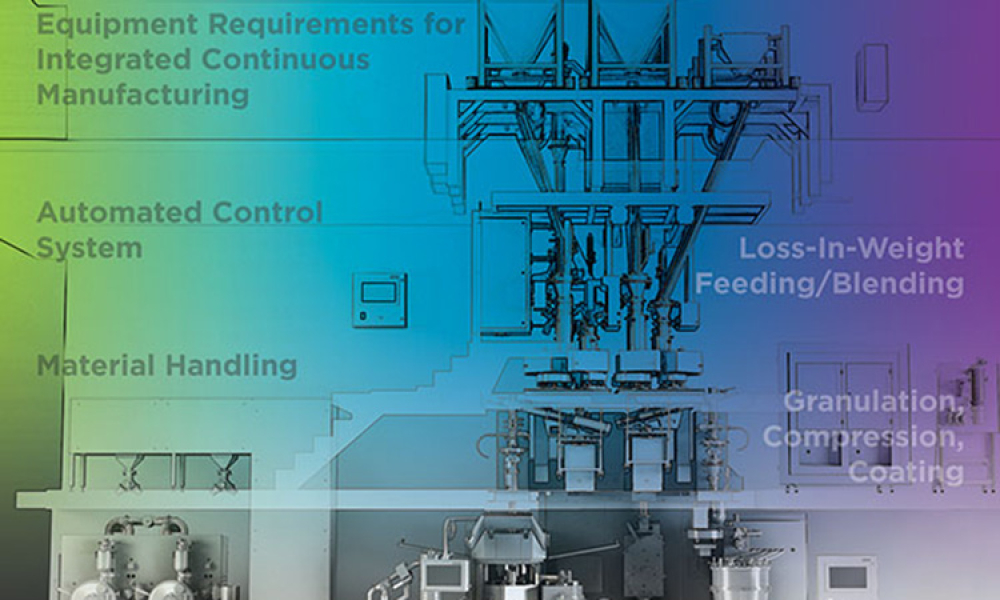
Oral Solid Dosage (OSD) is the inspection and evaluation of the manufacturing and control processes used to manufacture solid oral dosage form pharmaceutical products.
Related Guidance Documents
Advanced Manufacturing (1)
Manufacturing Operations (2)
Microbiological & Viral Contamination Control (1)
Community Discussions
Community Discussions
Dec 20, 2024
Dec 19, 2024
GAMP®
Dec 18, 2024
Dec 16, 2024
Biotechnology
Containment
Dec 11, 2024
Data Integrity
Dec 04, 2024
Good Manufacturing Practice
Dec 04, 2024
GAMP®
Lifecycle Management
Related Pharmaceutical Engineering Magazine Articles
Concept and Discussion Papers
Process Validation Lifecycle for Packaging Oral Solid Dosage Forms
In August 2017, ISPE published the Discussion Paper “Overview of Packaging Validation for Drug…

Videos Related to Oral Solid Dosage
iSpeak Blog Posts
Training Courses Related To Oral Solid Dosage
GMP Sterile Pharmaceutical Manufacturing Facility Training Course
This course uses the second edition of the ISPE Baseline® Guide: Sterile Product Manufacturing Facilities and the FDA's Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing - Current Good Manufacturing Practice to provide an understanding of the key requirements and GMPs for sterile manufacturing facilities.
Concept and Discussion Papers
March / April 2024
Navigating the Asia Pacific Pharmaceutical Landscape for Global Impact Cover: The Asia Pacific…
Pharma 4.0 – Towards IT/OT Architectures for Prescriptive Maintenance
Introduction Faced with global competition and shrinking margins, (bio)pharmaceutical manufacturers…
Process Events for the Life Science Industry - Information Model Concept on OPC UA for Alarms and Audit Trails
Abstract Plug & play in general describes a piece of equipment that is ready to use upon connecting…