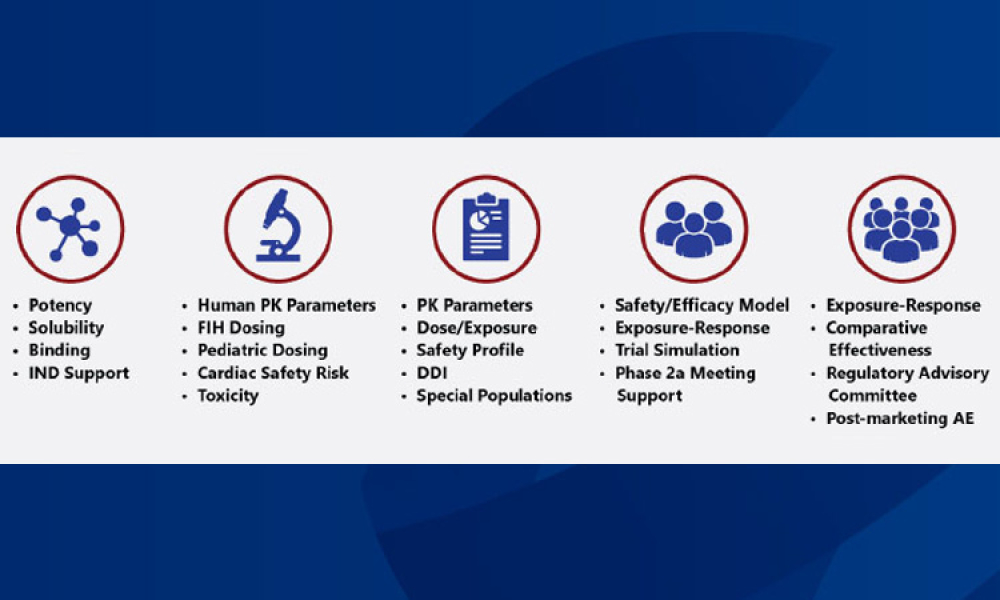
Investigational Products (Invest Prod) refers to a preventative (vaccine), a therapeutic (drug or biologic) device, diagnostic, or palliative used in a clinical trial. An investigational product may be an unlicensed or licensed product when used or assembled (formulated or packaged) differently from the approved form, when used for an unapproved indication, or when used to gain further information about an approved use.
Related Guidance Documents
Biotechnology (1)
Investigational Products (8)
- Comprehensive Guide to Clinical Materials
- Good Practice Guide: Comparator Management
- Good Practice Guide: Development of Investigational Therapeutic Biological Products
- Good Practice Guide: IMP Reverse Logistics
- Good Practice Guide: Harmonizing the Definition and Use of NIMPs
- Good Practice Guide: Clinical Supply Systems
- Good Practice Guide: Interactive Response Technology
- Introductory US Clinical Trial Materials Training Guide
Supply Chain Management (1)
Pharmaceutical Engineering Magazine Articles
Concept and Discussion Papers
Investigator Initiated Trials (IIT) – Considerations and Guidance from the Perspective of Clinical Trial Supplies and GMP
1 Introduction Conducting a clinical trial is a complicated process because of the many factors to…
Validation & Data Integrity in eClinical Platforms
1 Introduction It is well understood that each clinical trial is conducted and managed as an…
GAMP 5: Implementation & Operation of GxP Compliant Clinical System
1 Introduction The principles and processes for the validation of computerized systems are well…

Pharmaceutical Job Board
iSpeak Blog Posts Related to Investigational Products
Featured Conferences