Content focuses on the financial impact of data management systems on drug development, manufacturing, and distribution; the basic computer system life cycle model and the activities and software quality assurance practices in each phase; and the controls and methods necessary to maintain data integrity and security.
Guidance Documents
Advanced Manufacturing (1)
+Commissioning & Qualification (1)
+Data Integrity (15)
+GAMP® (14)
+Knowledge Management (1)
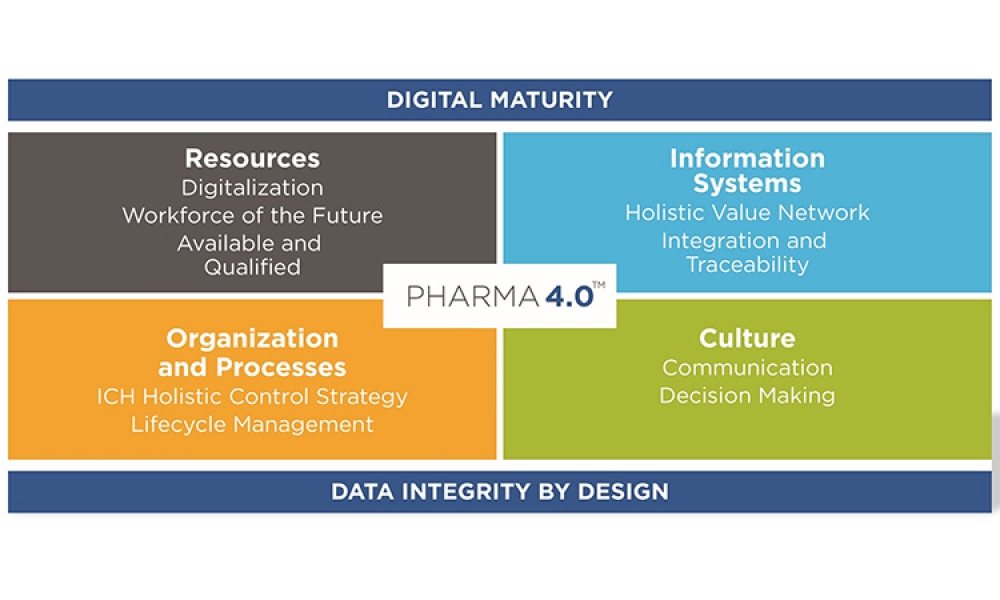
+Pharma 4.0™ (1)
+Regulatory (1)
+Validation (11)
+Community Discussions
Community Discussions
Apr 08, 2025
Validation
Apr 08, 2025
Data Integrity
Apr 07, 2025
Advanced Manufacturing
Biotechnology
Mar 28, 2025
Information Systems
Regulatory
Advanced Manufacturing
Active Pharmaceutical Ingredients
Mar 20, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Mar 20, 2025
Mar 20, 2025
Pharmaceutical Engineering Magazine Articles
White Papers
January / February 2024
Stakeholders across industries are becoming accustomed to using information technology (IT) systems…
March / April 2022
This article aims to refresh information on open-source software (OSS) within regulated computerized…

Webinars
Upcoming
On-Demand
None Available
ISPE in the News
Latest
-
ISPE Webinar: QRM Based Integrated C&Q Series – Good Engineering Practices (GEP) as a Key Enabler for Integrated C&Q
-
Sustainability Track - Building a Sustainable Future: A Pharmaceutical Engineering Perspective
-
Antiviral Chewing Gum Reduces Influenza, Herpes Viral Loads
-
EMA Seeks Input on Manufacturing mRNA Vaccines
-
Tariffs on Canadian drugs could cost US $750M
-
Siemens to Acquire Dotmatics
-
Schneider Electric Enhances Environmental Data Sharing
-
SHL Medical's $220M S.C. Facility to Create 300 Jobs
-
Critical Processing Zones Key to Novel Aseptic Processing Area Design
- Siemens to Help Miko Pharma Build Pharma Manufacturing Plant in Ghana