Quality is the strongest force multiplier for any organization’s growth. Attaining higher quality levels has a multi-dimensional effect throughout any organization. Creating and sustaining a passion for quality brings higher levels of responsiveness, confidence and flexibility to organization wide manufacturing operations. Overall quality and manufacturing data as represented by effective...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
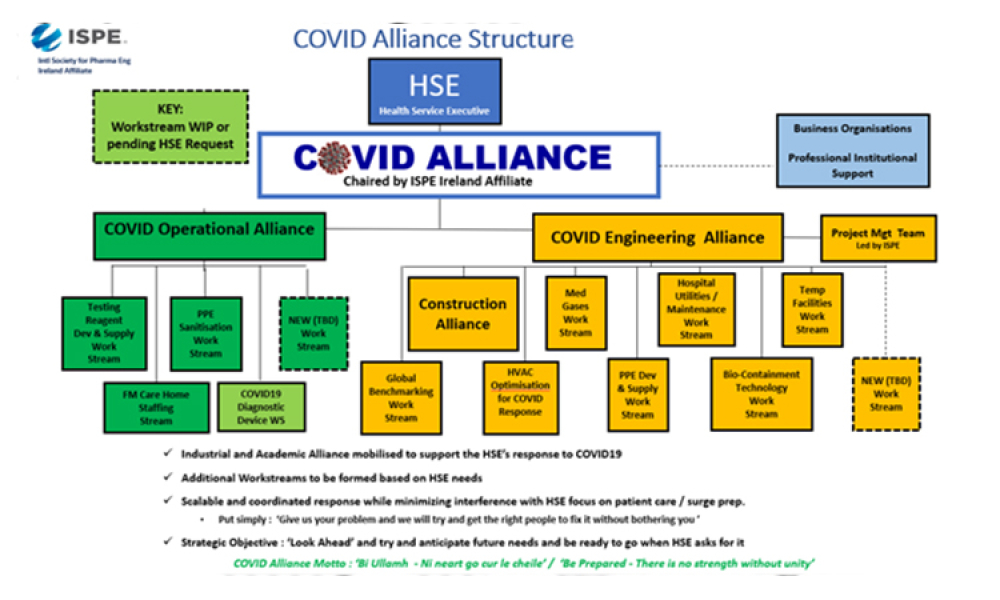
Early in March 2020, many around the world viewed the sight of collapsing intensive care units in Northern Italy, overwhelmed by COVID19, with great anxiety and we wondered how we might address the inevitable arrival of the virus at our doorsteps. This concern led to a home-grown initiative in Ireland that brought together representatives of health, commercial and academic organisations...
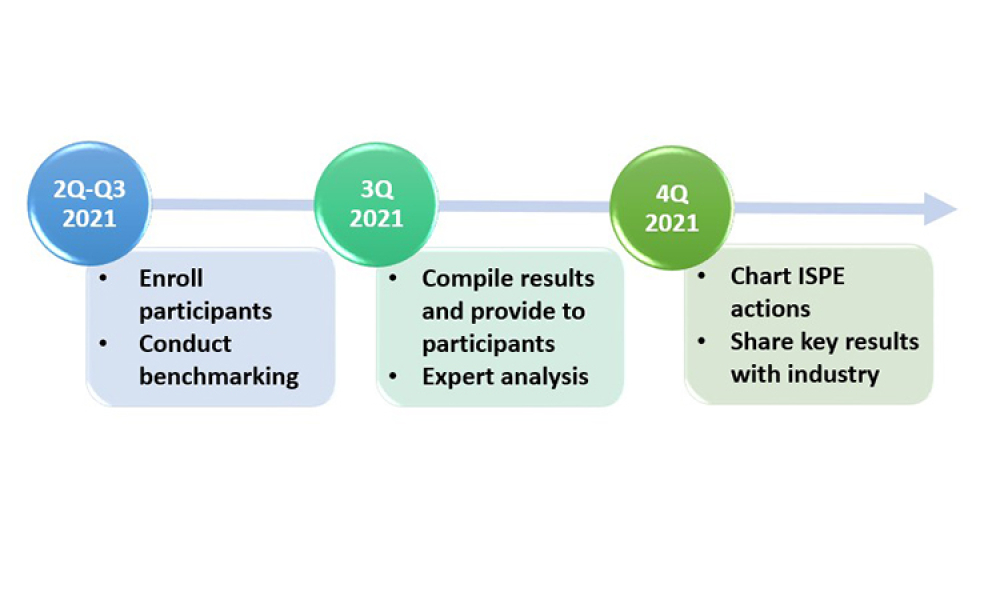
ISPE is pleased to announce that its Drug Shortages Initiative Task Team is launching a new phase of work to focus on business continuity planning for the prevention of drug shortages. We invite your company to participate. The first activity is a benchmarking study that will launch in May 2021 and be used to assess best practices and industry accomplishments in the area of business continuity...
You are an active and engaged ISPE Member! You sit on the ISPE Board of Directors, the ISPE Foundation Board, and the Steering Committee of Women in Pharma®. Can you share...
Prathiba Sampath, PMPFor me, both personally and professionally, 2020 started off as an incredible year. My husband and I were expecting the arrival of our first child in April. At work, I was being entrusted with increasing responsibilities and being groomed for a promotion, while having an incredible learning journey. I’d taken the lead on the development project for a new manufacturing...
The UK Entered a New Relationship with the European Union on 1 Jan 2021
The United Kingdom (UK) (England, Northern Ireland, Scotland and Whales) is no longer part of the European Union (EU) Customs Union. Simply put, it means movement of goods between the UK and the EU are now to be an import and export trade transaction. Multiple industries have been impacted, including...
Featured in this edition of the Pharmaceutical Engineering Online Reading Roundup are the most read online articles during March 2021. Learn more about understanding cleanliness classifications, steam sterilization, and more from what visitors to the PE Online site were reading last month.
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from March 2021. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
You have been an ISPE Member for 30 years! During that time, you have done so much for the Society (thank you!). Currently, you are the Co-Chair of ISPE Women in Pharma® and serve as a Board Member for the ISPE International Board of Directors and the ISPE Foundation Board. You have also worked with and Chaired numerous committees in the past. Can you share some of...
As the third (already!) month of 2021 is rapidly coming to its close, it is time for a recap and a look ahead. In the spirit of March being Women’s History Month, we’re taking this opportunity to focus on the women in our organization. Here’s a reflection on and celebration of some exciting past and future accomplishments for 2021, with women in the driver seat.
While I consider myself a long-time entrepreneur, the journey to begin my own consulting business started in Seattle over dinner with two colleagues. We were talking about what we want to do in our individual lives and sharing ideas about what those lives might look like. We discussed how our dreams could fit into what we each want to share with the world and the way the world is moving – so...
The past, present, and future were featured in the Opening Plenary Session at the 2021 ISPE Aseptic Conference. A record number of attendees at the virtual conference hear remembrances of the 30 years of the...
What Does Contract Manufacturing Look Like In Pharma?
Many factory productions can be outsourced to contract manufacturers (CMs) in order for businesses to continue providing quality products to their customers in a cost-effective way, and so they can focus on other aspects of their business, increasing their overall productivity and efficiency, and improving their bottom...
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from February 2021. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are the most read online articles during February 2021. Learn more about cleaning classifications, steam sterilization, biopharma process validation and more for what visitors to the Pharmaceutical Engineering® Online site were reading last month.
The current COVID-19 pandemic has heightened the awareness and attention to virus safety and risk mitigation for manufacturers. Each manufacturer has a strategy in place to mitigate the risk, however by manufacturers working together, this can lead to synergies and best practices in the way that the risk is managed. Also, additional guidance is required by the Industry in a field that is...
On February 8, 2021, the ISPE Emerging Leaders Committee launched ISPE’s first virtual International Hackathon. 51 ISPE Student and Recent Graduates participated in the event; they were split into six teams and 14 ISPE Emerging Leaders and Industry Professionals served as dedicated coaches to the teams.
On February 8, 2021, the ISPE Emerging Leaders Committee launched ISPE’s first virtual International Hackathon. 51 ISPE Student and Recent Graduates participated in the event; they were split into six teams and 14 ISPE Emerging Leaders and Industry Professionals served as dedicated coaches to the teams.