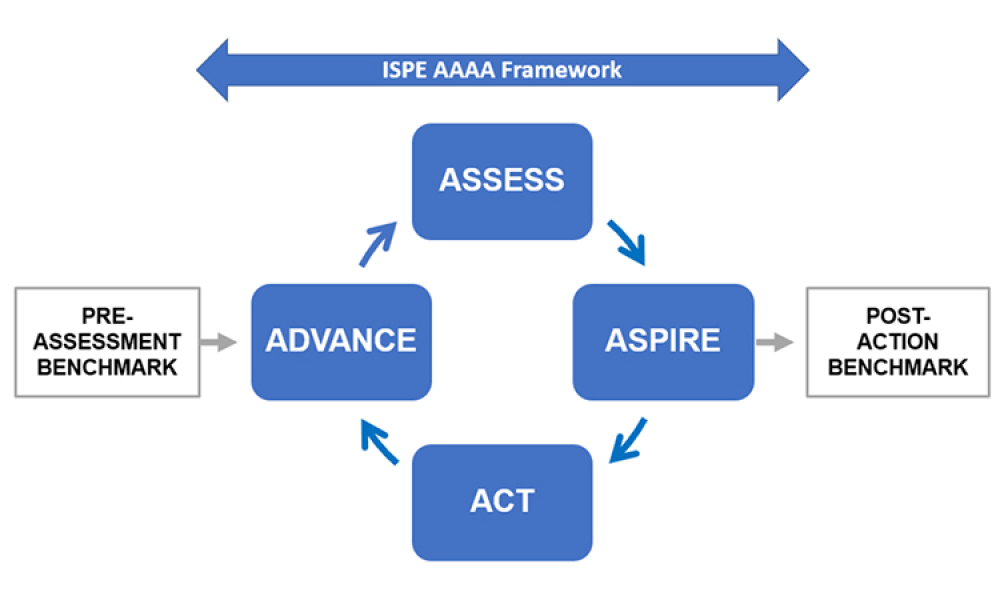
Featured in this edition of the Pharmaceutical Engineering® Online Reading Roundup are a look back at past winners of the Roger F. Sherwood Pharmaceutical Engineering Article of the Year Award.
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
This week ISPE released The ISPE GAMP® Good Practice Guide: Enabling Innovation – Critical Thinking, Agile, IT Service Management. The first such guidance to discuss...
Pharmaceutical Engineering® magazine is proud to announce the 2019 Roger F. Sherwood Article of the Year is “
Government Pharmaceutical Organization is the 2021 Facility of the Year Awards Winner for Social Impact for their Biological Produce (Vaccine) Production Plant in Saraburi Province,...
Janssen Sciences is the 2021 Facility of the Year Awards Winner for Project Execution for their BioCork2 – Large Scale Fed Batch Facility...
The needs of patients must always be at the forefront of what we do.
Many of our colleagues are taking on the challenge of rethinking the old ways. They are collaborating with each other and the regulatory agencies to take effective steps to remain at the forefront of regulatory science and innovation. Why? In order to ensure the continued delivery of effective medicines.
The San Francisco Bay Area Chapter has won numerous ISPE Awards by virtue of their contributions to the pharmaceutical industry. They continue to have their finger on the pulse of the local industry and respond with innovative and educational programs. The Chapter’s Emerging Leaders Committee is...
Takeda Pharmaceuticals International AG is the 2021 Facility of the Year Awards Winner for Process Intelligence & Innovation for their F36 New Solid...
Does your company only focus on how to get the product to patient without considering what happens if product needs to be retuned?
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from August 2021. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
ISPE is a global industry leader in scientific, technical, and regulatory advancement throughout the entire pharmaceutical lifecycle. As a result, ISPE is in a strong position and available to assist the US government, its allies, and like-minded regulatory partners with implementation of recommendations emerging from a report,
Imagine a time where medicines are made at your doorstep, where an illness can be treated in moments, storage conditions are a non-issue, quality is built in, and supply chains don’t matter. Your COVID-19 vaccine is manufactured, tested, and available for dosing, all within blocks of your home. Welcome to the age of transportable manufacturing.
ElevateBio is the 2021 Facility of the Year Awards Winner for Operational Excellence for their BaseCamp facility in Waltham, MA, USA.
It’s hard to believe we are more than halfway through 2021 and how 2020 is such a distance memory yet so ingrained into our minds it feels like it was yesterday. As COVID continues to be prevalent in society and with our continued focus on life saving medicines we as an industry must maintain our “Agility, Collaboration, Innovation”. The facility and engineering track would like to invite you...
Dr. Ferdinando E. Aspesi has been a member of ISPE for more than 29 years. Currently the Chair of the Pharmaceutical Engineering committee, he is also a member of the Delaware Valley Chapter and has been a member of the Global Pharmaceutical Manufacturing Leadership Forum, the PQLI Science and Technology Committee, and the Quality Metrics Team.
Takeda Pharmaceuticals International AG is the 2021 Facility of the Year Award Winner for Facility Integration for their Grange Castle P2 Facility in Grange Castle, Ireland.
This year’s ISPE Annual Meeting & Expo will be a combination of in-person presentations and events with virtual access to the presentations for those who cannot yet travel. The importance of information systems for our industry has evolved at an even faster rate. Digitalization is more important than ever, enabling us to work remotely effectively when needed and facilitates business...
All it takes is a quick Google search for the terms “carbon footprint and the pharmaceutical sector” to reveal some depressing reading. Results are led by a staggering piece of research from 2019 that states the global pharmaceutical industry is not only a significant contributor to global warming, but it also produces 13% more carbon emissions making medicines than car manufacturers do while...