Read, Learn, Innovate: Top iSpeak Blog Posts of 2019

Featured in this edition of iSpeak Reading Roundup are the top 10 blog posts from iSpeak in 2019. Gain key insights on FDA process validation guidelines, Pharma 4.0 developments, and more on what the pharmaceutical industry has explored and accomplished this year.
Continued Process Verification, 3rd Stage of FDA Process Validation Guidelines

Explore a plan for continued process verification and the crucial parameters and attributes necessary for successful GMP bioprocess. This plan at its core evaluates close attention to statistical accuracy and managing process vulnerabilities.
How GMPs & Data Integrity Align for Safer Products & Swifter Approvals

Discover the FDA’s additions to their guidance for Good Manufacturing Practices (GMPs) and the important requirements that companies need to adhere to in order to pass inspections successfully. This article features insights for data integrity which is a main area within GMP compliance.
What Should Pharma Expect When ICH Q12 is Implemented?

See the ICH Q12 regulatory guideline, featuring detailed instruction for the break-down of complex regulation and other challenges facing the pharmaceutical industry. Professionals can adjust current production and develop new operations for excellence with the support of these management strategies.
Pharma 4.0 – The New Frontier for the Pharma Industry

The pharma industry is ever-changing and developing new ways to move forward with science and technology. Pharma 4.0 incorporates advanced technology to promote innovation and to provide more reliable and secure data. Learn how new technology and operations will propel the industry forward.
2019 Pharma Best Practices – ISPE Launches New Webinar Series
ISPE has launched unlimited access for members to a brand-new webinar series including presentations from industry experts on crucial topics of interest in pharma manufacturing. Learn how to gain access to the series.
Common Pitfalls During Implementation of a Cleaning Validation Program

Risk for contamination and regulatory violations decrease with an analysis of common mistakes and redesigned maintenance procedures. Read about the necessary planning, pre-requisites, and challenges that interfere with approved cleaning validation practices.
Direct to Patient: The Basics & Quality Considerations

This article focuses on a plan for improving the patient experience in clinical trials by aiming to deliver products directly to their homes and to increase accessibility to certain drug therapies. The model seeks to help patients in clinical trials by educating suppliers and manufacturers on regulation and this plan’s initiative.
RIP Spreadsheets and Fishbones: Their Time Has Come and Gone
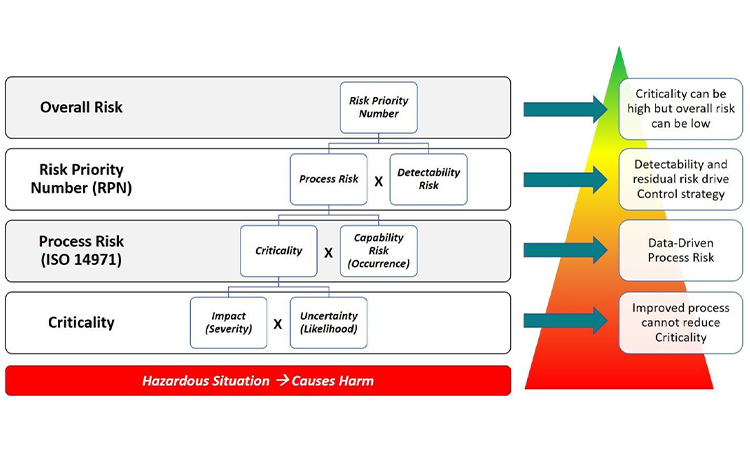
Industry tools for risk management and data-based decision making have become more progressive with the use of visual process mapping and multidimensional datasets. Learn more about how to leverage these tools in the presentation of quality data for pharmaceutical and medical devices in today’s facilities.
Data Integrity for Manufacturing Records

Hear from Charlie C. Wakeham on professionally reviewed instruction featured in the ISPE GAMP® Data Integrity for Manufacturing Records guide. The guide focuses on data Integrity and the importance of critical thinking for protection of laboratory data and records at every stage of the product lifecycle.
The Facility Challenges of Developing Continuous Process based Biopharmaceutical Products

Learn more about the impact of continuous manufacturing in biopharmaceutical manufacturing organizations. The Multi-Purpose Facility (MPF) plan is featured in this article, to educate the industry on this product development resource because it can adapt to a wide variety of batch or continuous processes leading to more efficient manufacturing.



