2019 Pharma Best Practices – ISPE Launches New Webinar Series
ISPE is excited to announce a complimentary webinar series, featuring leading subject matter experts covering critical topics in pharmaceutical manufacturing. Thought leaders will share their insights during a 45-minute presentation and then you’ll have the opportunity to get your questions answered during a 15-minute Q&A.
ISPE members have the benefit of unlimited access to the webinar recordings—even if you don't sign up for the webinar. Nonmembers that attend a webinar will have access to the recordings for 30 days. So, if you’re not a member—join today!
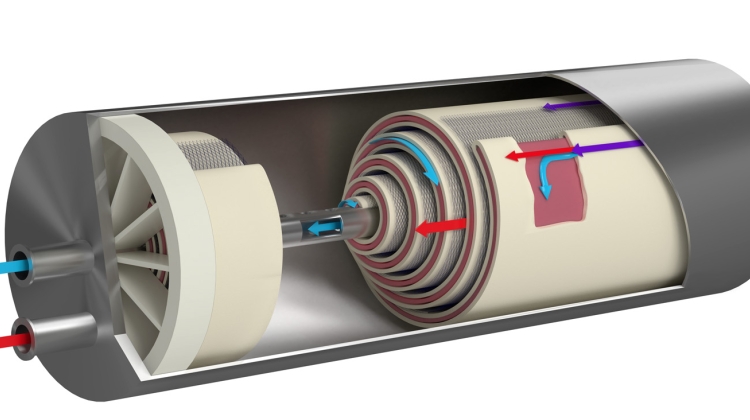
This new series kicks off on 29 April 2019 with the webinar Water for Injection Using Non-Distillative Methods – ISPE D/A/CH Approach. Presenter Fritz Röder, Senior QA Manager Validation, Qualification and Engineering Merck KGaA, Darmstadt, will share collective insights from the ISPE D/A/CH Affiliate (Germany/Austria/Switzerland) for Water and Steam, and provide answers to questions like: What are suitable technologies for the final treatment step of a cold WFI system?
Webinar: Water for Injection Using Non-Distillative Methods – the ISPE D/A/CH Approach
Since April 2017, the European Pharmacopeia has allowed pharmaceutical companies to produce Water for Injection using methods other than distillation. Meanwhile, the additional water quality standard for "Highly Purified Water", currently found in the Ph. Eur., will be removed in April 2019. This means huge changes in regulatory requirements for pharmaceutical companies, and although a new Q&A document had been launched by the EMA, some topics remain unclear to users. The ISPE D/A/CH Affiliate Water and Steam community has extensively discussed their questions and issues over the last 1.5 years to help clarify these topics for you.
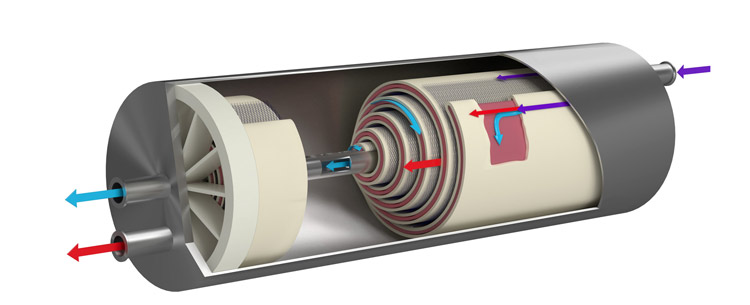
This webinar will provide insights into the thoughts of the ISPE D/A/CH Affiliate experts and additionally addresses the following questions:
- What are suitable technologies for the final treatment step of a cold Water for Injection system?
- How can the final treatment step be validated?
- How much control and oversight are needed for a cold Water for Injection system?
- Why did we set up a new standard to determine the cut-off of an ultrafiltration module?
- Help, High Purity Water does not exist anymore! Do I have to upgrade my existing HPW system now?
- How could a requalification from High Purity Water to Water for Injection be carried out?
Meet the Presenter:
Upcoming Webinars:
May 2019
New GAMP® Data Integrity Good Practice Guidance and Experience from the Field
Presenters: Sion Wyn, Director, Conformity Ltd. and Paul Moody, Director Global Engineering Projects, Allergan Pharmaceuticals Ireland
June 2019
GAMP® Good Practice Guide Guide for GxP Compliant Lab Computerized Systems
Presenters: Mark Newton, Principal, Heartland QA, and Paul Smith, Global Strategic Compliance Program Manager, Agilent Technologies




