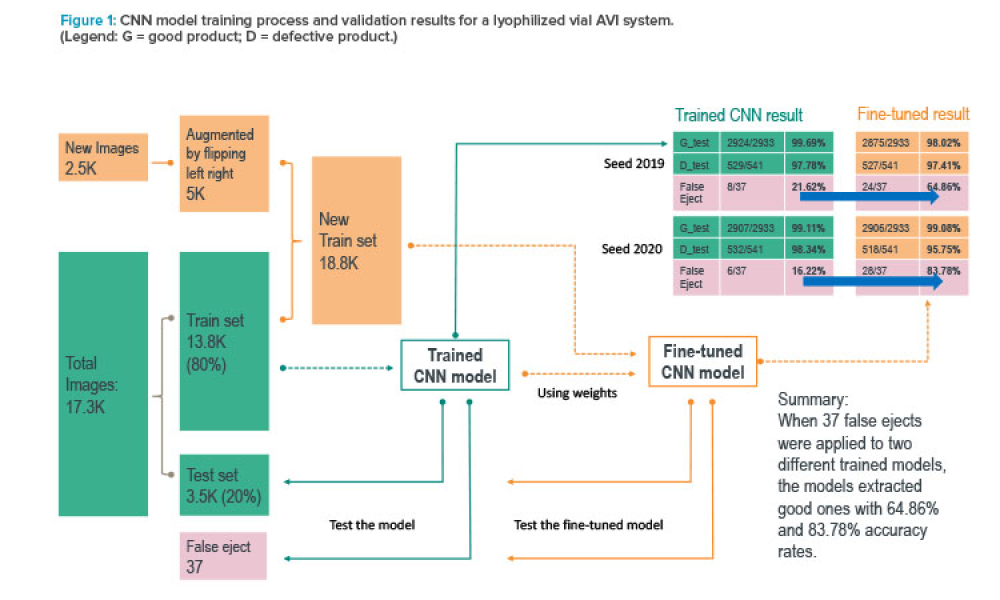
Containment (Containmt) is the action of confining within a defined space a microbiological agent or other entity that is being cultured, stored, manipulated, transported, or destroyed to prevent or limit its contact with people and the environment. Methods to achieve containment include physical and biological barriers and inactivation using physical or chemical means.1.Primary Containment. Addresses the protection of personnel and the immediate laboratory environment from exposure to infectious agents. It involves using closed containers or biological safety cabinets along with secure operating procedures.
Related Guidance Documents
Containment (4)
Microbiological & Viral Contamination Control (3)
Quality by Design (1)
Sustainability (1)
Sustainable Facilities, HVAC, & Controlled Environments (2)
Community Discussions
Community Discussions
Jan 30, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Jan 28, 2025
Jan 27, 2025
Jan 27, 2025
Regulatory
Regulatory
Jan 27, 2025
Information Systems
Jan 27, 2025
Regulatory
Regulatory
Jan 27, 2025
Good Manufacturing Practice
Sustainable Facilities, HVAC, & Controlled Environments
Pharmaceutical Engineering Magazine Articles
Webinars Related to Containment
Featured Conferences

Videos Related to Containment
iSpeak Blog Posts Related to Containment
Training Programs
GxPs for Leadership
This comprehensive course will equip you with the knowledge and skills to ensure GxP compliance and inspection readiness in the pharmaceutical industry. You will thoroughly understand regulatory requirements and learn about management's role in maintaining safety and quality. The course covers essential topics such as regulatory expectations, responsibilities in compliance, and consequences of non-compliance.
GMP Refresher
Code of Federal Regulations (CFR) states that "Training in current good manufacturing practice shall be conducted by qualified individuals on a continuing basis and with sufficient frequency to assure that employees remain familiar with cGMP requirements applicable to them." This course will provide an overview of History, Regulations, Quality Systems, and development, with a focus on distinguishing between QA and QC in GMP. It can serve as a GMP refresher for both individuals and companies and ensures that current key trends are covered. CEUs are provided once you achieve an 80% passing grade…
Advancing Pharmaceutical Quality (APQ) Quality Management Maturity Training Course
The ISPE Advancing Pharmaceutical Quality (APQ) Program has been developed by industry representatives, for industry use, to provide a practical framework that organizations can use to assess and advance the state of quality within their organization. The APQ program recognizes that the ability to advance the maturity of quality management lies within the industry itself and provides a range of sustainable and practical quality management improvement strategies.
GMP Fundamentals: Eleven-Part Bundle Series
Obtain a 10% Savings by Purchasing All Eleven Courses Overview ISPE is presenting an eleven-part series that will focus on the fundamentals of good manufacturing practices (GMPs). The series provides an overview of the regulations pertaining to GMPs and covers topics such as: manufacturing controls, product distribution, plant hygiene, documentation practices, buildings & facilities, organizational structure, and more. This offering gives users access to all eleven modules of the series and is intended to introduce GMPs for the new pharmaceutical employee or to provide an annual refresher for…
CAPA / RCA / Investigations Training Course
Coming Soon 2024 - CAPA and Continuous Improvement using Process Performance & Product Quality Monitoring (PPPQMS), are elements of the Pharmaceutical Quality System (PQS), supported by ICH Q10. By practicing effective CAPA and PPPQMS a Pharmaceutical Quality System can realize Quality Management Maturity.