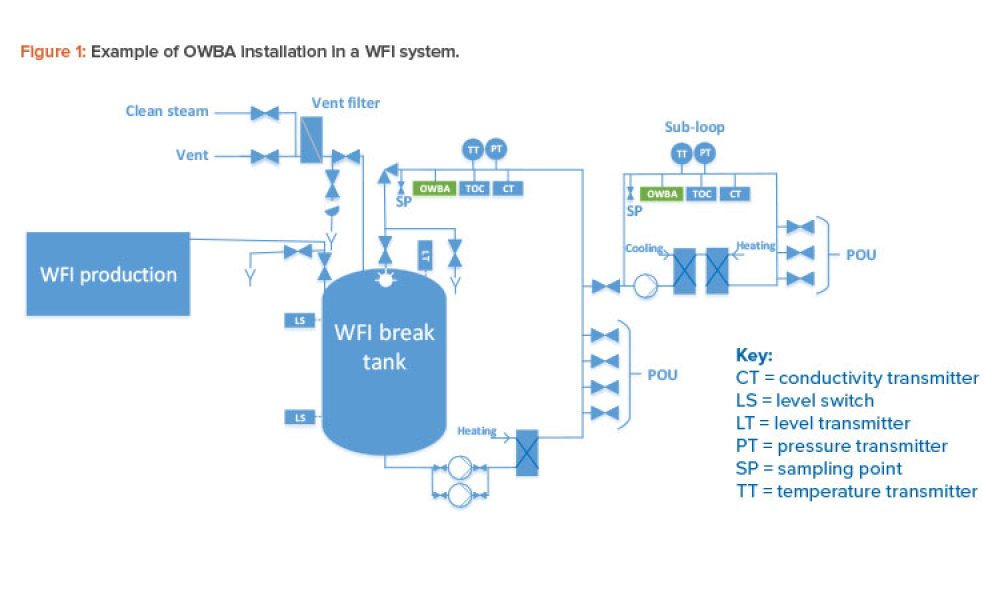
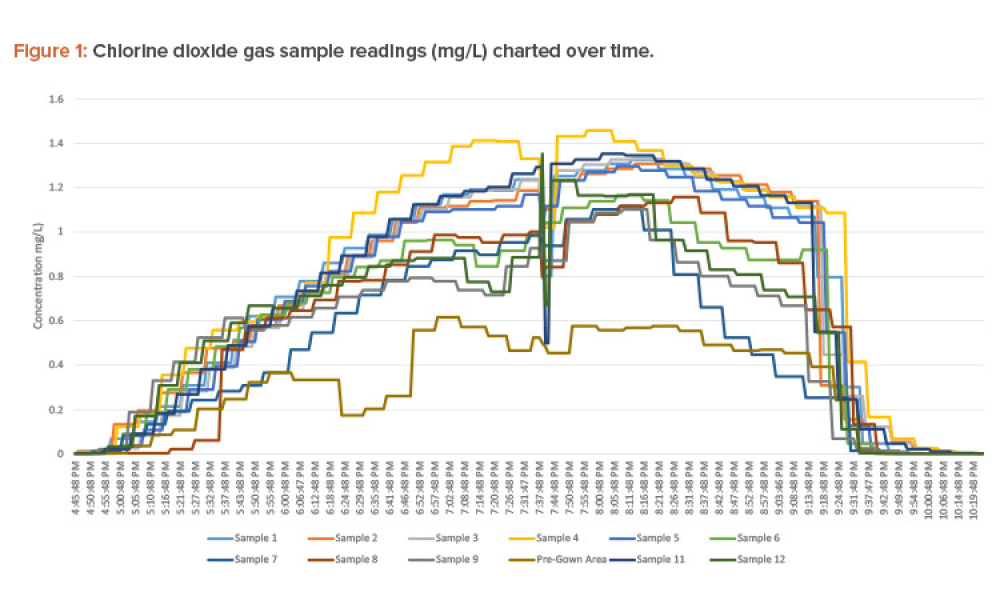
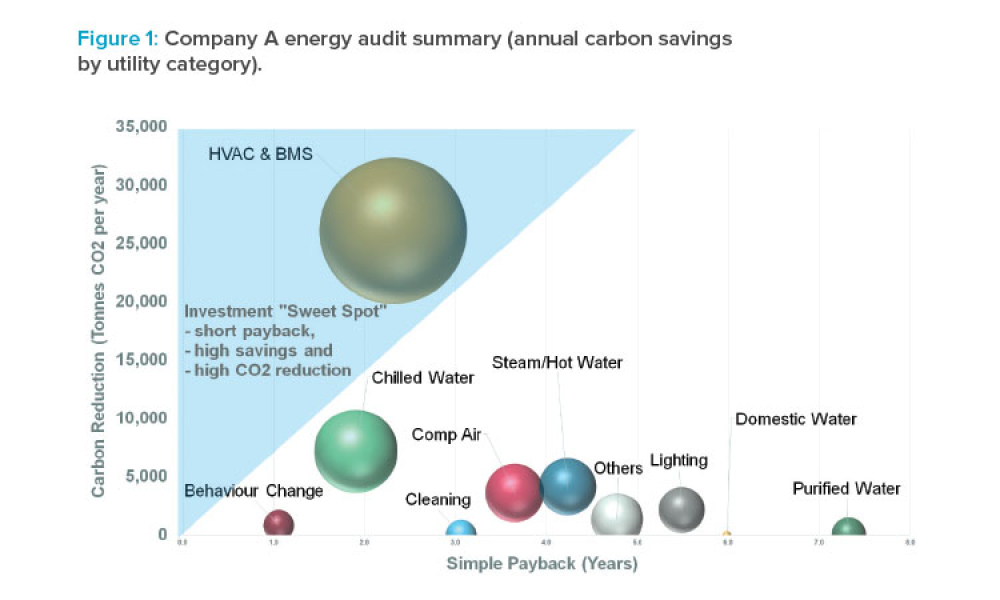
Critical Utilities (Crit Util) like water and HVAC (Heating, Ventilation, and Air Conditioning) systems form the backbone of the manufacturing process. As a result, these are treated, as products that must satisfy FDA regulatory requirements and pharmaceutical manufacturing standards, just like raw materials and other equipment used in the industry.
Related Guidance Documents
Commissioning & Qualification (1)
Critical Utilities (8)
- Good Practice Guide: Process Gases 2nd Edition
- Baseline Guide Vol 4: Water & Steam Systems 3rd Edition
- Good Practice Guide: C&Q of Pharma Water & Steam Systems 2nd Edition
- Good Practice Guide: Ozone Sanitization of Pharma Water Systems
- Good Practice Guide: Membrane-Based WFI Systems
- Good Practice Guide: Critical Utilities GMP Compliance
- D/A/CH Affiliate: WFI Handbook (English Translation)
- Good Practice Guide: Sampling Pharma Water, Steam, & Process Gases
Microbiological & Viral Contamination Control (6)
- Good Practice Guide: Process Gases 2nd Edition
- Baseline Guide Vol 4: Water & Steam Systems 3rd Edition
- Good Practice Guide: Ozone Sanitization of Pharma Water Systems
- Good Practice Guide: Membrane-Based WFI Systems
- D/A/CH Affiliate: WFI Handbook (English Translation)
- Good Practice Guide: Sampling Pharma Water, Steam, & Process Gases
Quality Control (1)
Pharmaceutical Engineering Magazine Articles
Webinars Related to Critical Utilities
iSpeak Blog Posts Related to Critical Utilities
Training Programs Related To Critical Utilities
Critical Utility GMP Compliance
This course is focused on how to be compliant in the design, operation & qualification of critical utilities and how to prove it. It comprises four modules including interactive case studies from real experience. This helps the participants to experience live discussions on compliance topics. The content is primarily based on the new ISPE Good Practice Guide “Critical Utilities GMP Compliance”.
Storage, Delivery, and Qualification Pharma Water Training Course
This course will explore the essential concepts and principles of specification, design, commissioning/qualification of equipment and systems used to store and distribute water in pharmaceutical manufacturing. The course has been substantially updated to feature the guiding principles of the ISPE Baseline® Guide: Water and Steam Systems (Second Edition) with particular emphasis placed upon the new chapters for microbial control, laboratory water and rouging.
Pharmaceutical Water Systems
This course has been substantially updated to feature the guiding principles of the ISPE Baseline Guide: Water and Steam Systems (Second Edition) with particular emphasis placed upon microbial control and laboratory water as well as key design philosophies. The principles of design and operation of water systems used directly in pharmaceutical manufacturing and laboratory applications, including the essential concepts and principles of systems used to generate USP, EP and non-compendial waters will be covered.
Pharma Water Generation USP WFI & Purified Water Training Course
This course will cover the principles of design and operation of water systems used directly in pharmaceutical manufacturing and laboratory applications, including the essential concepts and principles of systems used to generate USP and non-compendial waters. These concepts include specification, design, operation, testing, and maintenance of equipment and systems for water generation.
Pharmaceutical Job Board
Videos Related to Critical Utilities
Featured Conferences