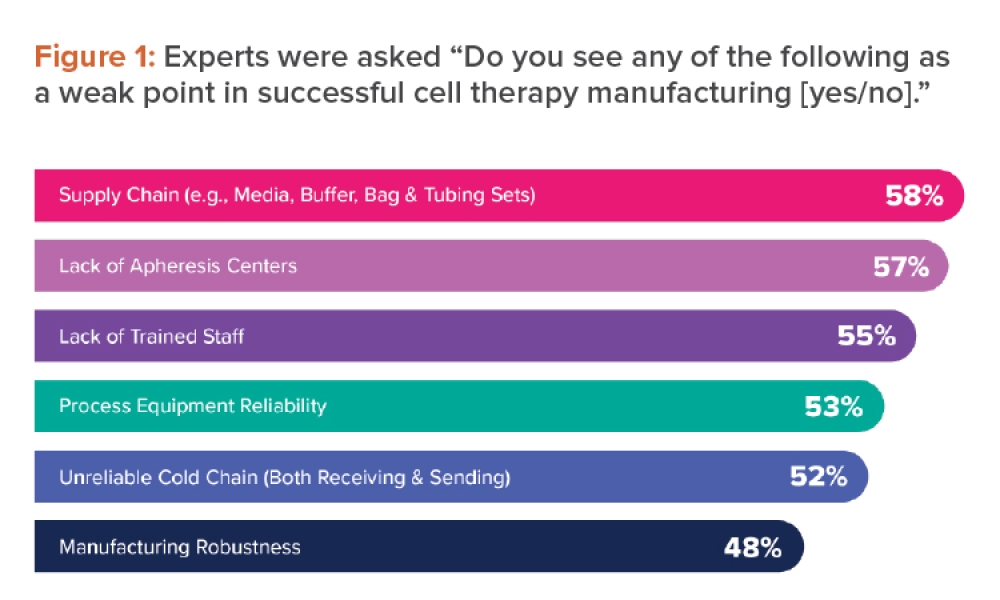
Advanced Therapy Medicinal Products (ATMPs) are medicines for human use based on genes, tissues, or cells. They offer groundbreaking new opportunities for the treatment of disease and injury.
Related Guidance Documents
Advanced Therapy Medicinal Products (1)
+Biotechnology (1)
+Manufacturing Operations (1)
+Community Discussions
Community Discussions
Feb 01, 2025
Jan 31, 2025
Sustainable Facilities, HVAC, & Controlled Environments
Jan 30, 2025
Jan 27, 2025
Jan 27, 2025
Regulatory
Regulatory
Jan 27, 2025
Information Systems
Jan 27, 2025
Regulatory
Regulatory
Webinars Related to Advanced Therapy Medicinal Products
Upcoming
On-Demand
None Available
iSpeak Blog Posts Related to Advanced Therapy Medicinal Products
Pharmaceutical Engineering Magazine Articles
Videos Related to Advanced Therapy Medicinal Products
Training Courses Related to Advanced Therapy Medicinal Products
Biopharmaceuticals: Webinar C> e ATMP
+On Demand Biopharmaceutical Three-Part Bundle Series
+Biopharmaceuticals: C> and ATMP
+Biopharmaceuticals: C> and ATMP
+ATMP Manufacturing
+Pharmaceutical Job Board
Featured Conferences