This article discusses how blockchain technology may disrupt the way we collect and manage data within regulated processes. The first section is a nontechnical summary of blockchain’s features, including a description of what it is (and what it is not). This sets the context for the next section, in which we discuss several blockchain use cases currently being piloted by life sciences...
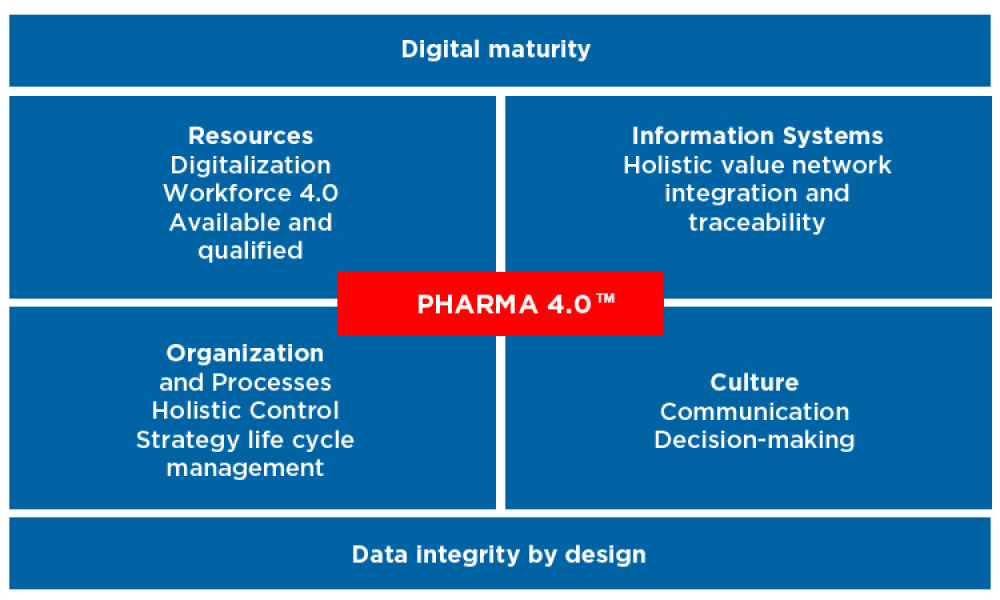
Information Systems
The Fourth Industrial Revolution could completely transform health care.
The amount of data collected in a typical pharmaceutical manufacturing operation is staggering, yet research shows that much of this information is rarely used for anything more than compliance. New technologies such as big data, artificial intelligence, machine learning, and deep learning permit unprecedented analysis of realtime data and can even predict trends in processes and operations....
ISPE's revised IT Infrastructure Control and Compliance Guide provides comprehensive guidance on regulatory expectations for both traditional and cloud-based IT platforms. Have we done enough?
Recent cyberattacks like WannaCry and Petya have affected GxP computerized systems, prompting questions on how to address risk from cyberspace using traditional computerized systems validation according to GAMP® 5. This article explores life cycle management of GxP computerized systems and associated cybersecurity risks that can affect patient safety.
The digital revolution is driving change across all industries. With its ability to increase transparency and trust between parties, the recent innovation called blockchain has the potential to significantly disrupt the clinical trials industry.