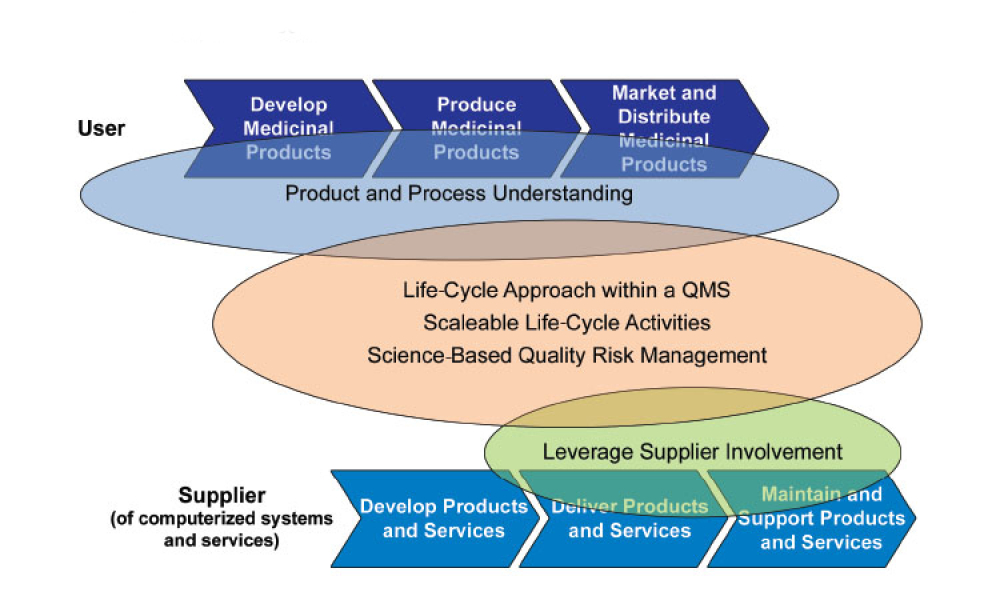
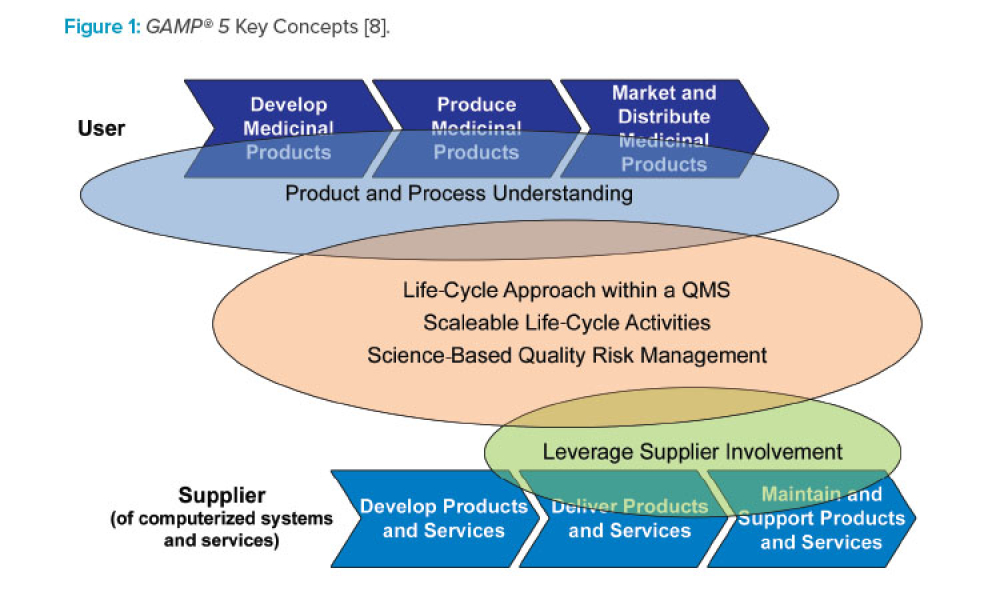
GAMP® 5
Features
The US FDA Center for Devices and Radiological Health (CDRH) Case for Quality program promotes a risk-based, product quality–focused, and patient-centric approach to computerized systems. This approach encourages critical thinking based on product and process knowledge and quality risk management over prescriptive documentation-driven approaches.