Over 2,600 attendees from across 30 countries and a remarkable 200 exhibitors convened at the 2023 ISPE Annual Meeting & Expo in Las Vegas, NV, USA.
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
Close to 500 people celebrated the 2023 Facility of the Year Award (FOYA) Winners at a reception and banquet Sunday night, 15 October, during the 2023 ISPE Annual Meeting & Expo at Mandalay Bay in Las Vegas, Nevada, USA. Genentech was announced as the 2023 FOYA Overall Winner.
A fireside chat featuring Robert M. Califf, MD, FDA’s 25th Commissioner of Food and Drugs, and Thomas B. Hartman, President and CEO of ISPE kicked off the 2023 ISPE Annual Meeting & Expo. The following is a brief summary of the session. Look for complete coverage of the interview in an upcoming issue of Pharmaceutical Engineering® magazine.
Through the ISPE Foundation Diversity Internship Program, junior undergraduate and graduate-level students are afforded an opportunity to immerse themselves in a summer of groundbreaking engineering projects at some of the most cutting-edge facilities. This experience not only fosters the development of invaluable technical skills, but also serves as a first chance for students to establish a...
As the 2023 ISPE Annual Meeting & Expo in Las Vegas, NV, USA draws near, anticipation builds among the 110 students and Emerging Leaders from around the world who will be in attendance. These exceptional...
The 2023 Facility of the Year Awards (FOYA) Honorable Mention recognizes projects that did not win a specific category but were clearly successful projects that overcame significant challenges in planning, execution, and delivery.
The 2023 Facility of the Year Award (FOYA) Pharma 4.0™ category recognizes companies that embody the Pharma 4.0™ concept. This includes not only implementing one or more technological innovations, but also demonstrating the ability to change their culture, processes, and people orienting them towards a 4.0 future....
The countdown has begun, and the anticipation is evident as we gear up for the highly anticipated 2023 ISPE Annual Meeting & Expo. As we look back at the successful conferences over the past years, this event promises to be nothing short of extraordinary, especially for students and Emerging Leaders.
At ISPE, our commitment to advancing the pharmaceutical industry has always revolved around delivering gold-standard Guidance Documents — a trusted source of information that’s been a helping hand for professionals like you to rely on when forging new paths and exploring uncharted territory . In the ever-evolving landscape of pharmaceutical and scientific advancements, we understand that how...
The 2023 Facility of the Year Awards (FOYA) Social Impact category recognizes companies that accelerated a shift to sustainable facility design, intended to ensure the effective use of energy, minimize waste, reduce carbon footprint, incorporate green manufacturing techniques, and reduce environmental...
Few industries have as deep a footprint in California, USA as life sciences. The sector is home to nearly 14,000 businesses that employ more than a million people at an average wage of more than $162,000 – Californians employed in life sciences earn a staggering $54.6 billion a year. And despite uncertainties in the US economy and massive layoffs in the high-tech sector, California life...
For several years, ISPE has steadfastly organized hands-on training sessions to enrich classroom learning. Despite the challenges posed by the pandemic, we have successfully developed a program that integrates practical, experiential learning, leveraging ISPE's comprehensive training materials. We are thrilled to announce our partnership with the European Aseptic and Sterile Environment (EASE)...
PIC/s Annex 1, and the WHO Annex 2 for the manufacturing of sterile products took effect on 25 August 2023, and the time to attend the 2023 ISPE Pharma 4.0™ and Annex 1 Conference couldn’t be better. The Equipment Innovation and Annex 1 Implementation Track Co-leads Richard Denk and Matthew Gorton and the Emerging Leader Co-lead Pol Bonet invite you to join them for this exciting event in...
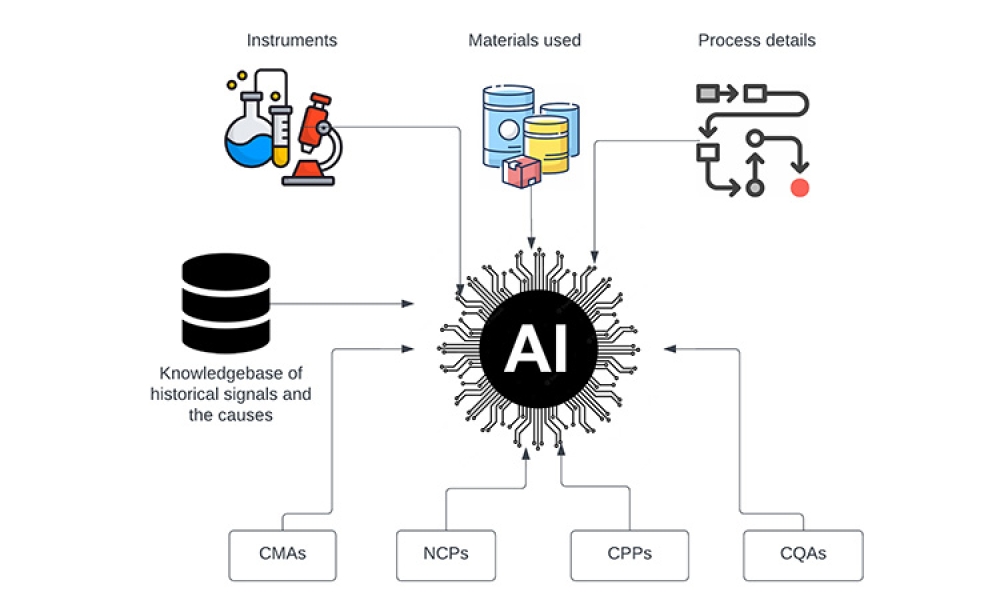
The pharmaceutical industry is constantly evolving, placing a significant emphasis on emerging technologies that facilitate quicker access of medications for patients globally. This unceasing drive for innovation and improvement has led the industry to embrace cutting-edge technologies like data analytics, machine learning, and artificial intelligence. These technologies are not just trends;...
With a recent increase in interest and participation, the ISPE GAMP® Special Interest Group (SIG) focusing on Manufacturing Execution Systems (MES) has mapped out an ambitious number of subject areas to use as the basis for future publications, presentations, and best practice guidance documents. With the co-leadership of Christian Wöelbeling, Executive Industry Advisor at Körber Pharma...
Facility of the Year Award (FOYA) Winners in the Supply Chain category exemplify novel application of principles, systems, and management tools aimed at improving operational speed, robustness, and response. These could be applied to areas in which delivery to market impacts patients, technology amplifies supply...