The 2023 ISPE Aseptic Conference will devote one entire day to case studies related to aseptic manufacturing projects. For us, seeing the real-life challenges that the industry is facing and how project teams...
Submit Your Best Content to ISPE
ISPE’s official blog, iSpeak accepts contributions from our Members and professionals in the pharma industry.
ISPE is pleased to announce the complete Advancing Pharmaceutical Quality Program (APQ) Program is now available with the release of the fifth and final guide.
By the ISPE Regulatory Team
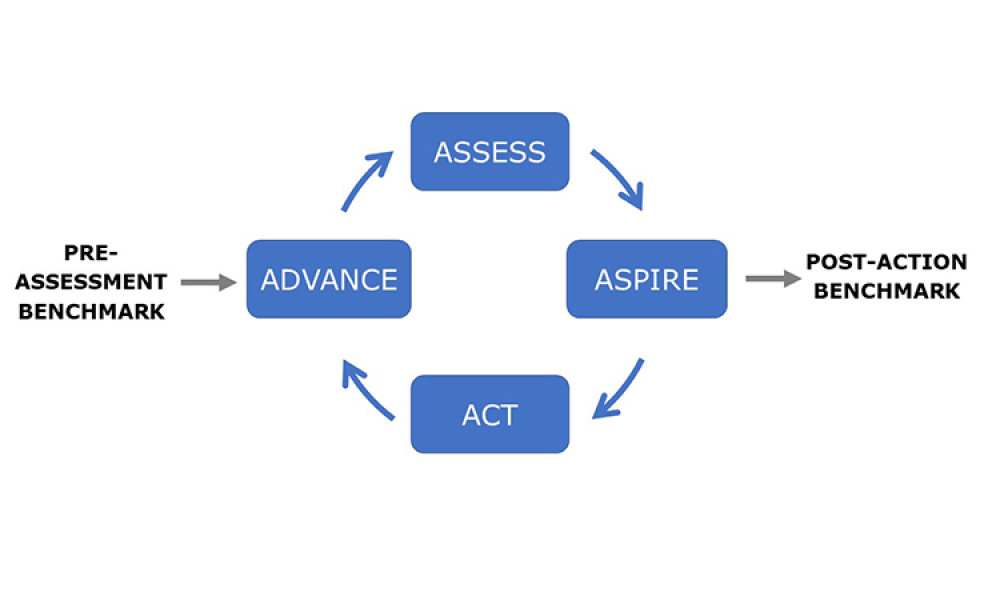
ISPE’s regulatory volunteer groups are charged with bringing visibility and solutions to major challenges faced by the industry with regard to regulatory/quality matters, and facilitating the flow of information between ISPE members and global Health Authorities. Support for that mission was on display at the 2022 ISPE Annual Meeting and...
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) celebrated its 50th anniversary at a special symposium in Dublin, Ireland on 4 October 2022. Since the first meeting of the PIC Committee in 1972, PIC/S has come a long way! The symposium was titled “Thriving at 50 and Striving Forward” and was aimed at promoting and...
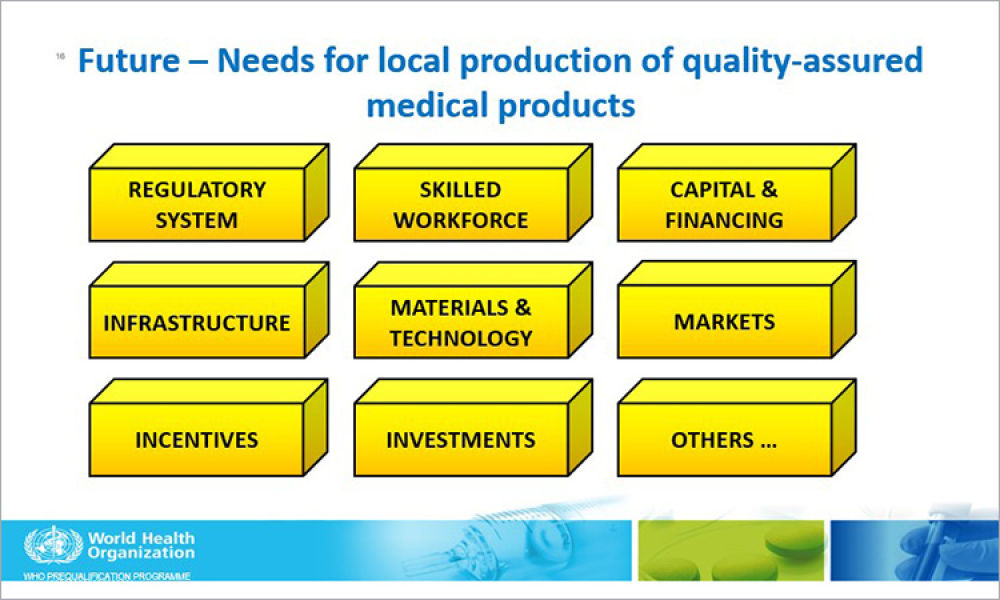
As we are all aware, the COVID-19 pandemic has had a unique, world-wide effect on the pharmaceutical industry as well as health authorities. At the height of the pandemic, and continuing as it appears to wane, health authorities have not been able not carry out normal, in-person inspections of pharmaceutical manufacturing sites. However, in the ”Age of Covid,” using the Internet and associated...
ISPE Aseptic is one of those conferences where the depth of knowledge astounds me. When I started attending and speaking at the ISPE Aseptic Conference in the 2000s, I came to grow and learn.
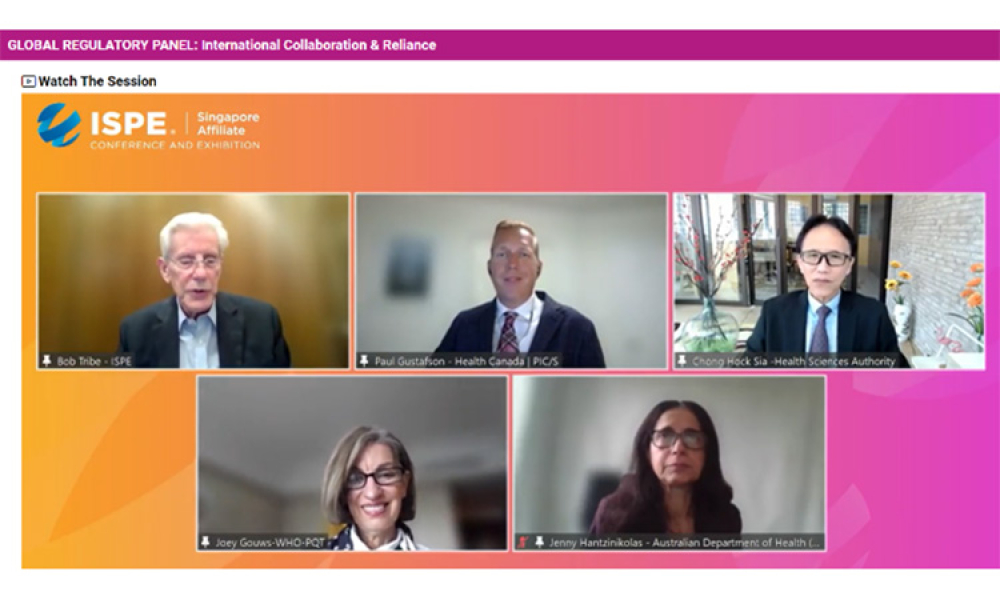
The 2022 ISPE Singapore Affiliate Conference & Exhibition featured a regulatory roundtable discussion on the Future of Pharmaceutical Inspections.
On 17 August 2022, four international regulators discussed how the Covid-19 pandemic had taught regulators to look for alternative ways of doing GMP inspections and also to rely more on each other to make determinations on the GMP status of manufacturers of medicines.
On the 1st of June'22 a Career Workshop took place in the European City of Science'22 – Leiden, the Netherlands. This workshop was organized by the ISPE NL EL Community of Practice (CoP) within the Life Science & Health Career Event hosted by
The 2022 ISPE International Emerging Leader Hackathon officially started on Saturday 29 October 2022 at 0800 EST, but the connections started happening the night before during the Hackathon Reception at Wreckers Bar and Grill in the Gaylord Palms Resort, Orlando, FL USA.
The regulatory keynote and the Global Regulatory Town Hall panel discussion at the 2022 ISPE Annual Meeting & Expo on 2 November provided insights into regulations and the changing scene with increased...
The gavel passed to a new International Board Chair and ISPE leaders shared 2022 achievements and plans for 2023 at the 2022 ISPE Membership Meeting and Awards Lunch.
The industry is in the process of a transformation that was sped up by the pandemic, which enhanced innovation and collaboration. Applying what has been learned over the last three years to update pharmaceutical companies’ approaches to development and implementation is key as the industry crafts its strategies in an increasingly digital and global environment. The keynote presentations on 1...
The 2022 ISPE Annual Meeting & Expo opened with three keynote addresses exploring quality from a key FDA leader’s point of view, a major pharmaceutical company’s ongoing journey of transformation into an organization that is prepared to adapt to the changing world, and a unique patient perspective with a patient presenter who is also a member of the pharmaceutical community.
More than 200 people celebrated the 2022 Facility of the Year Award Winners at a reception and banquet Sunday night at the 2022 ISPE Annual Meeting & Expo at the Gaylord Palms in Orlando, Florida.
At the ISPE Singapore Conference on 17 August 2022 during an online panel discussion, four international regulators discussed how the Covid-19 pandemic had taught regulators to look for alternative ways of conducting GMP inspections and also to rely more on each other to make determinations on the GMP status of manufacturers of medicines.
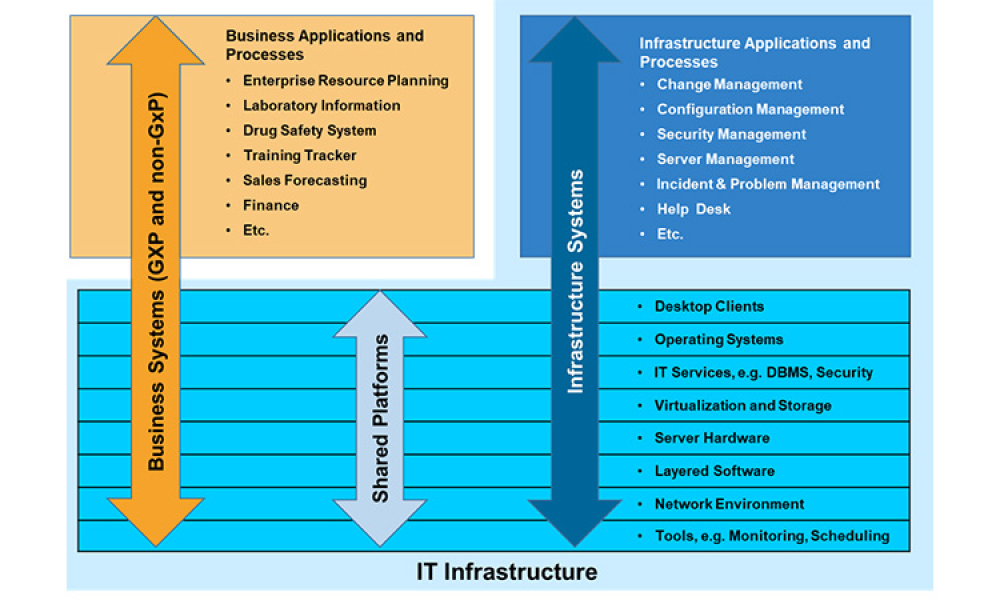
We are The Culture Club, a self-formed cross-CoP group of individuals who came together to discuss the quality/validation challenges encountered as we transition into the digital world and identify ways to influence cultural changes needed within our industry to better enable innovation. This blog is the first in a 4 part series intended to explore how we can rethink the way we support and...