Top Ten Pharmaceutical Engineering Magazine Articles of 2019

Featured in this edition of iSpeak Reading Roundup are the top 10 articles from the 2019 issues of Pharmaceutical Engineering® magazine. Discover key insights for data integrity, manufacturing excellence, innovative validation practices, and more for what the pharmaceutical industry has accomplished this year.
Biopharmaceutical Manufacturing Process Validation and Quality Risk Management
See key insights for the nature of continual process validation in today’s industry and a risk management approach to quality operations. This article delves into the evolution of process validation practices and offers step by step details for optimal efficiency in for this style of management strategy.
Data Integrity in the Trenches: A Look into Quality Control Lab
Discover regulatory rules and how the industry can better navigate complex restrictions. The pharmaceutical industry will benefit from this discussion of data integrity, quality control, and GMP inspections.
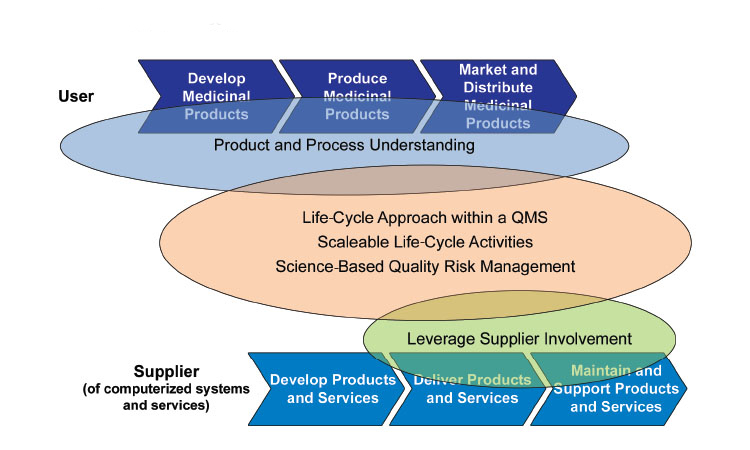
GAMP 5: Ten Years On
Ten years ago, the ISPE GAMP® 5 Guide: A Risk-Based Approach to Compliant GxP Computerized Systems, focuses on user and supplier initiatives for successful process understanding of data lifecycles and the impact of developing pharmaceutical products in the future.
New & Improved: Meet the Annex 1 Revisions
Explore the European Commission’s new revisions and additions to their annex 1 guideline and the incorporation of a risk management strategy for sustainable quality operations. Annex 1 focuses on innovative production for sterile manufacturing.
A Holistic Approach to Production Control
Read how the development of control strategies to commercial manufacturing through technology transfer will transform today’s industry and best practice methodology that would change the current operations into a holistic production control strategy.
Design & Control of Pharma Water System to Minimize Microbiological Contamination
Learn the importance of water purification in preventing drug interactions with other substances or ingredients in product formulation. This article features insights and crucial ideas for why these systems need to be monitored using microbiological study.
Blockchain for Pharmaceutical Engineers
Gain an understanding for ways your organization can leverage new technology with blockchain operations and equipment. This article reviews how blockchain technology may impact the way pharmaceutical facility managers collect and manage data within regulated processes.
Bowtie Analysis and Barrier-Based Risk Management
This article reviews the bowtie analysis approach and illustrates a simple demonstration of a risk management strategy for barrier control and containment. The bowtie approach highlights specific contaminants such as flammable liquids.
Risk Management in Single-Use Technology
See the implementation of a risk management strategy during implementation of single-use technology (SUT) and the involvement of new equipment and, in some cases, changes to an existing process. This article also focuses on the product lifecycle and the inclusion of the recent technological advancements.
Equipment Surfaces - Redefining Acceptable Conditions to Eliminate Unnecessary Maintenance
Discover the current approach to cleaning and operational maintenance of current facility equipment. This article features insights for avoiding unnecessary maintenance and how to increase efficiency through time management and standard operations.