
Featured in this edition of ISpeak Reading Roundup, are the top blog posts from August 2019. Discover key insights for data integrity, manufacturing excellence, innovative validation practices, and more for what the pharmaceutical industry was reading last month.
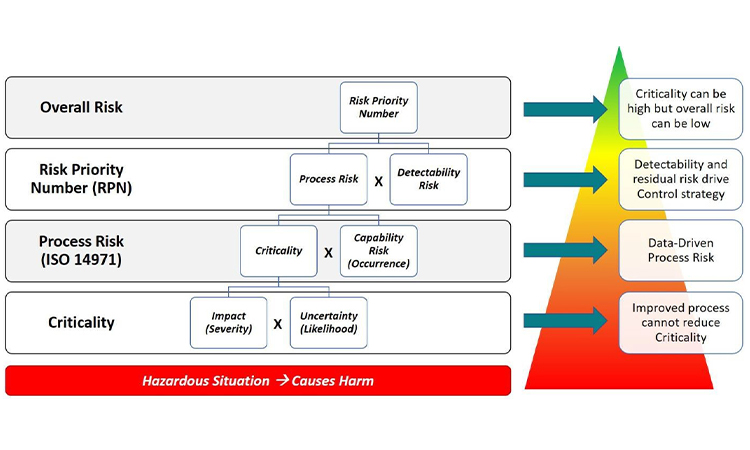
Industry tools for risk management and data-based decision making have become more progressive with the use of visual process mapping and multidimensional datasets. Learn more about how to leverage these tools in the presentation of quality data for pharmaceutical and medical devices in today’s facilities.

Hear from Charlie C. Wakeham on professionally reviewed instruction featured in the ISPE GAMP® Data Integrity for Manufacturing Records guide. The guide focuses on data Integrity and the importance of critical thinking for protection of laboratory data and records at every stage of the product lifecycle.

See the industry excel with Moderna, Inc. and their application of modern technologies, innovative solutions, and progressive facility concepts. Their new facility in Norwood, MA will help eliminate supply chain risks and solidify their commitment to patients in providing life-saving medicines.

Risk for contamination and regulatory violations decrease with an analysis of common mistakes and redesigned maintenance procedures. Read about the necessary planning, pre-requisites, and challenges that interfere with approved cleaning validation practices.
Explore a plan for continued process verification and the crucial parameters and attributes necessary for successful GMP bioprocess. This plan at its core evaluates close attention to statistical accuracy and managing process vulnerabilities.
The following blog post was provided by Peyton Myers, an undergraduate student at Appalachian State University. Myers attended the 2023 ISPE Annual Meeting & Expo in Las Vegas as an ISPE Foundation Professional Development Grant recipient.
The integration of data science in biopharmaceutical manufacturing, emphasizing data quality, tech transfer efficiency, and process optimization, is the heart of this track. Led by industry experts, discussions explore leveraging digital twins, predictive analytics, and continuous improvement initiatives. Additionally, interactive roundtable discussions provide attendees with a dynamic forum...
The pharmaceutical sector stands at a crossroads of immense possibilities. We are witnessing an unprecedented surge in innovation, fueled by a remarkable partnership between industry and regulatory authorities. For example, there are initiatives fostered by the US Food and Drug Administration (US FDA) to promote innovation with programs such as CATT (CBER Advanced Technologies Team) and ETP...