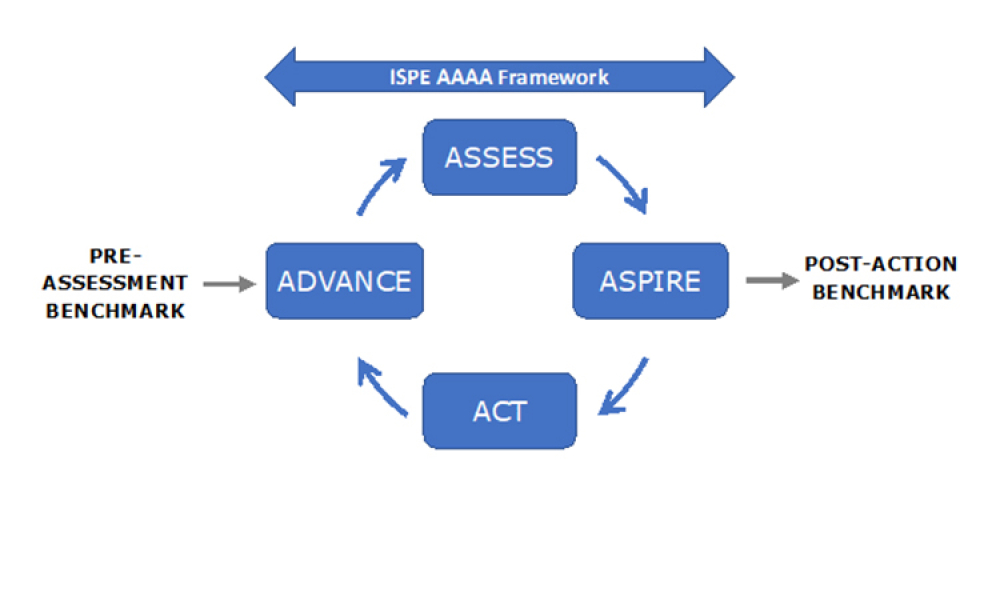
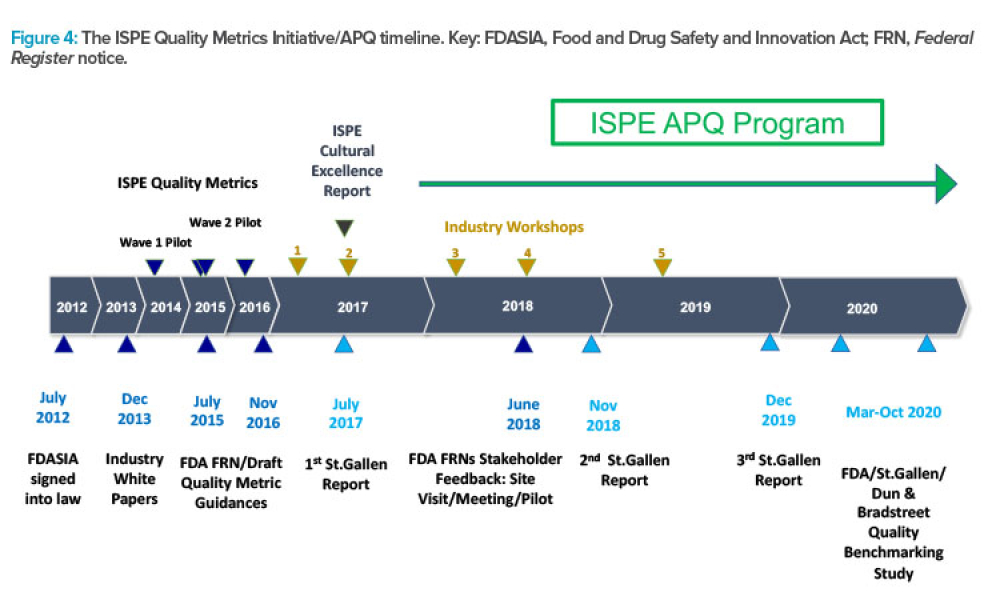
ISPE has announced the launch of its Advancing Pharmaceutical Quality (APQ) Program with the publication of the ISPE APQ Guide: Corrective Action and Preventive Action (CAPA)...
Christopher John Potter, PhD

Related Articles
2020 ISPE Europe Annual Conference
At the 2020 ISPE Europe Annual Conference on 16–17 September, which was a virtual conference, three regulatory panel discussions included more than 20 regulators...
This article is Part 2 of a two-part series exploring what we can learn from examples of pharmaceutical products being approved using accelerated programs. The series focuses on challenges that chemistry, manufacturing, and control (CMC) development teams may encounter when a project is given accelerated development status. In
This article is Part 1 of a two-part series exploring what we can learn from examples of pharmaceutical products being approved using accelerated programs. The series focuses on challenges that chemistry, manufacturing, and control (CMC) development teams may encounter when a project is given accelerated development status. Part 1 introduces key considerations and themes in general terms and...