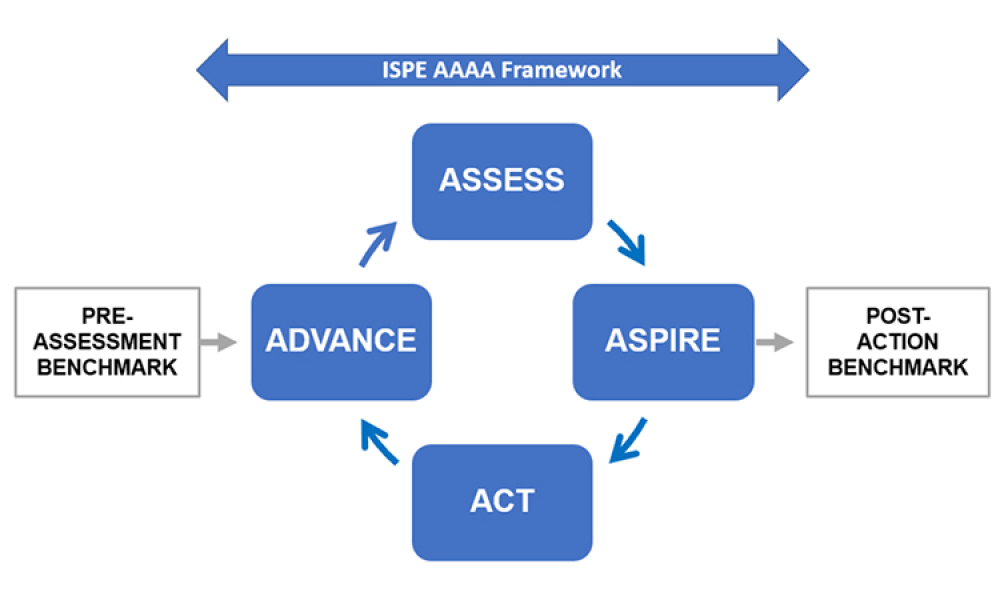
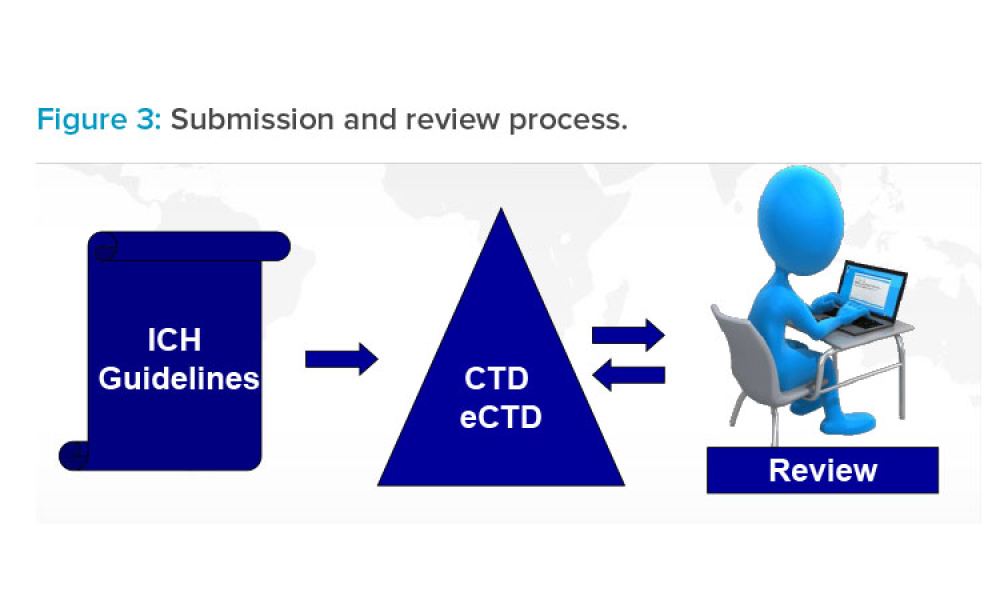
A panel discussion held on 27 April 2022 during the 2022 ISPE Europe Annual Conference focused on Digital Transformation.
Christopher John Potter, PhD

CMC Pharmaceutical Consultant
Chris Potter graduated from the University of Exeter with a degree in chemistry and completed a PhD at Imperial College London University in organic chemistry. He started work at Beecham Research Laboratories, and moved to Sterling-Winthrop to take management positions in both pharmaceutical and analytical development. During this period he worked on both ethical and over-the-counter drug development. For the later period of his career, Potter moved to ICI Pharmaceuticals, later Zeneca, then AstraZeneca where he had a senior position as manager of Analytical Development and R&D QA and CMC Project Management Group with responsibility in both the UK and US. He finished his career as Director of External Pharmaceutical Programmes. Potter retired at the end of October 2007 and is now performing part-time CMC consultancy work. He is currently part time Technical Project Manager for ISPE's PQLI Program. Potter was a member of EFPIA's ad hoc Quality Group from 1996 to 2007, and during this period was EFPIA topic leader for ICHQ6A, Specifications for New Drug Substances and New Drug Products, and ICH Q4B, Regulatory Acceptance of Pharmacopoeial Interchangeability. Potter led EFPIA's PAT Topic Group, which produced a Mock P2 to promote discussion and understanding regarding how ICH topics Q8 and ICH Q9 could be implemented.