Regulatory Progress - Global Advancement of Continuous Manufacturing for Pharma

Continuous manufacturing (CM) is an advancement in pharmaceutical manufacturing technology that provides high assurance of product quality as well as enough flexibility and agility in production to respond to market demands. The decision to invest in CM can be challenging for a company given the cost of purchasing the continuous equipment, the resources and expertise required for additional in-process monitoring and testing, and the automation complexity of the integrated system. However, the economic benefits afforded by CM, specifically from optimizing supply chains by manufacturing according to variable product demand, provide a strong justification for this investment. Furthermore, several regulatory agencies have recently approved solid oral dosage forms for multiple new chemical entities as well as an already approved and marketed product, and these approvals have generated significant interest in and excitement about this novel approach to manufacturing pharmaceuticals.
To date, products manufactured by CM have been approved in at least seven markets, with several applications under review. The US FDA alone has approved five different applications for solid oral dosage forms produced by CM (Table 1).1 , 2 It is particularly encouraging to note that regulatory agencies have approved a range of equipment designs, differing degrees of equipment integration, and a wide variety of control strategies with varied extent of in-process monitoring and controls and use of real-time release testing. From presentations at conferences, it is clear that regulatory agencies are reviewing each CM application based on its individual merit, using a science- and risk-based approach to assess the manufacturing process and the product characteristics. This nonprescriptive approach drives innovative, creative thinking and supports the continued growth of CM for small- and large-molecule applications.
| Products | Approval Date | Company | Application Type |
|---|---|---|---|
| Orkambi (lumacaftor/ivacaftor) | August 2015 | Vertex | New chemical entity |
| Prezista (darunavir) | April 2016 | Janssen | Marketed product |
| Verzenio (abemaciclib) | September 2017 | Eli Lilly | New chemical entity |
| Symdeko (tezacaftor/ivacaftor and ivacaftor) | February 2018 | Vertex | New chemical entity |
| Daurismo (glasdegib) | November 2018 | Pfizer | New chemical entity |
- 1Lee S. L. “Emerging Technology Program: A Key Enabler for Successful Adoption of Novel Pharmaceutical Technologies.” Presentation at the ISPE Continuous Manufacturing Workshop, Arlington, VA, 6–7 June 2018.
- 2Pfizer Inc. “U.S. FDA Approves Daurismo™ (Glasdegib) for Adult Patients with Newly- Diagnosed Acute Myeloid Leukemia (AML) for Whom Intensive Chemotherapy Is not an Option,” press release, November21, 2018. https://investors.pfi zer.com/investor-news/press-release-details/2018/US-FDA-Approves-DAURISMO-glasdegib-for-Adult-Patientswith-Newly-Diagnosed-Acute-Myeloid-Leukemia-AML-for-Whom-Intensive-Chemotherapy-is-Not-an-Option/default.aspx
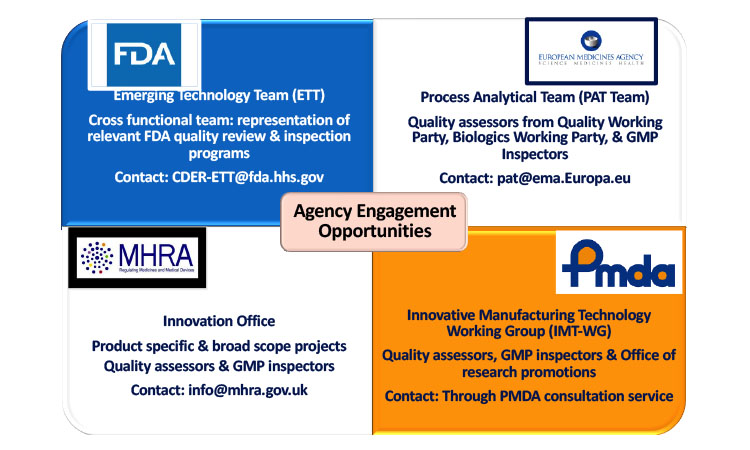
Team Approach
To support innovation in manufacturing and the adoption of novel technologies, several regulatory agencies have formed teams to advise sponsors seeking to implement new technologies such as CM. These regulatory teams include the Emerging Technology Team within the US FDA, the Process Analytical Technology Team within the European Medicines Agency, the Innovative Manufacturing Technology Working Group within Japan’s Pharmaceuticals and Medical Devices Agency (PMDA), and the Innovation Office within the United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA) (Figure 1).
These focused groups within each agency encourage companies to engage with agencies early and frequently as they work on new technology initiatives. The goal is to provide an opportunity to discuss novel strategies and approaches prior to the regulatory submission. This dialogue between industry and regulatory agency helps regulators understand the technology and alerts sponsors to potential regulatory concerns. Early engagement with regulators reduces regulatory uncertainty and lowers the number of questions during the review period. Each regulatory agency has a specific pathway to initiate interaction, and the opportunity to leverage these resources may be limited based on their availability and the agency’s priorities at the time.
Although current regulatory guidance does not prevent the use of CM, final guidelines on the topic are not yet available. The lack of regulatory guidelines can hinder technology implementation, regulatory approval, and life-cycle management for CM-manufactured products, especially when those products are intended for international markets.
ICH Q13
To address these concerns, CM was selected as a topic for the recently initiated International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline, “ICH Q13: Continuous Manufacturing of Drug Substances and Drug Products”.3
The ICH Q13 guideline under development seeks to harmonize CM-related definitions and regulatory concepts, dene key scientific approaches for CM, and clarify regulatory expectations. Some specific aspects of CM that the guideline is expected to address include state of control, system dynamics, material traceability, process models, and advanced process controls. The final document, which will likely be issued around 2021, will provide clarity on how drug manufacturers can use flexible approaches to develop, implement, and integrate CM for new and existing drug substances or drug products.
The ICH guideline process provides a unique opportunity to increase exposure to and knowledge of CM among regulators worldwide. ICH currently includes 10 regulatory agencies representing 37 countries and 13 regulatory observers, and it continues to grow steadily. Worldwide harmonization of regulatory concepts is especially important for CM because high levels of integrated measurements and controls are frequently used in CM processes. Alignment among all regulatory agencies on the different approaches for developing regulatory control strategies for CM is essential for the efficient deployment of these technologies.
As we wait for a finalized ICH guideline on CM, conferences and publications provide useful technical and regulatory information. Recently, conferences and workshops covering CM for both small and large molecules have proliferated; some of these events have been organized by ISPE as stand-alone conferences or as part of other regular conferences such as the ISPE Annual Meeting or the ISPE Facilities of the Future Conference. For additional regulatory insight into current approaches and expectations for CM, readers may refer to several recent publications on CM that are technical and regulatory/quality focused 4 , 5 , 6 , 7 , 8 as well as articles summarizing notable CM conferences., 9
- 3International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. “Final Concept Paper. ICH Q13 Continuous Manufacturing of Drug Substances and Drug Products.” 14 November 2018. https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q13/Q13EWG_ConceptPaper_2018_1115.pdf
- 4Allison G., Y. T. Cain, C. Cooney, T. Garcia, T. G. Bizjak, H. Oyvind, N. Jagota, et al. “Regulatory and Quality Considerations for Continuous Manufacturing May20–21, 2014, Continuous Manufacturing Symposium.” Journal of Pharmaceutical Sciences 104, no. 3 (March 2015):803–12. doi:10.1002/jps.24324
- 5Lee S. L., T. F. O’Connor, X. Yang, C. N. Cruz, S. Chatterjee, R. D. Madurawe, C. M. V. Moore, L. X. Yu, and J. Woodcock. “Modernizing Pharmaceutical Manufacturing: From Batch to Continuous Product.” Journal of Pharmaceutical Innovation 10, no. 3 (September 2015):191–9. doi:10.1007/s12247-015-9215-8
- 6Nasr M. M., M. Krumme, Y. Matsuda, B. L. Trout, C. Badman, S. Mascia, C. L. Cooney, et al. “Regulatory Perspectives on Continuous Pharmaceutical Manufacturing: Moving from Theory to Practice: September26–27, 2016, International Symposium on the Continuous Manufacturing of Pharmaceuticals.” Journal of Pharmaceutical Sciences 106, no. 11 (November 2017): 3199–206. doi:10.1016/j.xphs.2017.06.015
- 7Matsuda, Y. “Global Regulatory Landscape.” AAPS PharmSciTech 20, no. 1 (December 2018):2. doi:10.1208/s12249-018-1230-x
- 8Hernán, D. “Continuous Manufacturing: Challenges and Opportunities. EMA Perspective.” Slide presentation at the 3rd FDA/PQRI Conference on Advancing Product Quality 22–24 March 2017. http://pqri.org/wp-content/uploads/2017/02/3-DHernan.pdf
- 9International Society for Pharmaceutical Engineering. “ISPE 2016 Continuous Manufacturing Conference Highlights.” Pharmaceutical Engineering 37, no. 3 (May–June 2017): 35–42.
Acknowledgment
The author would like to thank Christine Moore, Global Head and Executive Director GRACS CMC-Policy at Merck & Co., Inc., Kenilworth, New Jersey, US, for reviewing the article.


