Corrosion Investigation of Pharma Clean Steam Systems

This article presents current research on the problem of rouge in clean steam generators and their distribution systems, as well as possible deleterious effects on capital equipment and final drug products.
Pharmaceutical clean (pure) steam systems consist of a generator, distribution tubing or piping, thermodynamic or balanced pressure thermostatic traps, control valves, pressure- reducing regulators, pressure gauges, pressure-relief valves, and volumetric totalizers. Most of these components are made of 316L stainless steel and contain fluoropolymer gaskets (most commonly polytetrafluoroethylene, also known as PTFE or Teflon), as well as semimetallic or other elastomeric materials. These components tend to corrode or degrade in service, potentially compromising the quality of the final clean steam (CS) utility product.
This project investigated stainless steel coupon samples from four CS system case studies, testing condensate for metals and particles, and conducting a risk assessment of potential corrosion effects on process and critical utility systems. Examining the corrosion byproducts involved preparing sample coupons of corroded tubing and components from distribution systems.9
These case studies investigated a variety of surface conditions, and included analysis of typical rouge products and corrosion effects. The referenced sample surfaces were evaluated for rouge deposits by visual inspection, scanning electron microscopy (SEM), auger electron spectroscopy (AES), and electron spectroscopy for chemical analysis (ESCA)/x-ray photoelectron spectroscopy (XPS). These techniques reveal the physical and atomic properties of the corrosion and deposits, and identify potential contributions to the critical utility fluid properties or final product.1
Overview
Stainless steel corrosion products are encountered in a variety of forms, such as a ferric oxide rouge layer (red or brown) on the metal surface found under- or overlying the thicker ferrous oxide layer (dark gray or black).2 The rouge layer is crystalline in structure and potentially dynamic, or capable of migrating downstream. The ferrous oxide (black rouge) layer tends to thicken over time as the deposit becomes more pronounced; its migratory presence is evidenced by particles or deposits found on sterilizer chamber surfaces and on equipment or vessels after steam sterilization. Laboratory analyses of condensate samples illustrate the particulate nature of the rouge as well as the level of soluble metals in the CS fluid.4
Water quality plays a major part in rouge product chemistry
While there are multiple causes of these phenomena, the CS generator is often a significant contributor. It is not uncommon to notice ferric oxides of rouge (red/brown) on the surface, with ferrous oxides (gray/black) at the steam discharge, with both types slowly migrating throughout the CS distribution system.6
The CS distribution system is a branching configuration that has multiple use points, terminating at distant areas or ends of a main header and various branching subheaders. The system may include a series of regulators to reduce pressure/temperature at certain use points; these may be sites for corrosion. Corrosion can also occur in hygienically designed traps placed at various points within the system to remove condensate and air from the mobile clean steam, in downstream piping/tubing to drains, past the traps, or in condensate collectors. Reverse migration is evident in most cases, with rouge deposits forming above the traps and growing upstream into adjacent use point piping or into subheaders and beyond; the rouge that forms in traps or other components is found upstream from this source and continues to migrate both upstream and downstream.
Rouge in steam systems can be found in all forms including:
- Class 1: migrating rouge that forms in one place and migrates to another surface
- Class 2: rouge that forms on the surface where the corrosion occurs
- Class 3: rouge formed in higher-temperature conditions (over 95°C)10
At use points, ball valves or valve housings exhibit significant rouge accumulation. Certain stainless steel components also demonstrate moderate to high levels of a disparate metallurgical structure, including delta ferrite. Ferrite crystal structure is suspected of lowering corrosion resistance, even though its content may only be 1%–5%. In addition, ferrite does not possess the corrosion resistance of austenitic crystal structure, therefore, it will corrode preferentially. Ferrite can be detected accurately with a ferrite meter or semi-accurately (and with significant limitations) using a magnet.
SUMMARY
From system inception, when a new CS generator and distribution tubing is first commissioned and energized, several potential factors for corrosion are present:
- In addition to clean steam, the CS generator begins to generate corrosion particles (class 1 rouge) that have the potential to migrate.
- Separately, pressure regulators begin to generate (class 3) rouge downstream, and possibly upstream as a function of time.
- High levels of delta ferrite, metallic inclusions, or other material defect content in components begin to generate corrosion products (class 2 rouge).
- Condensate traps can add further migration-capable corrosion (class 1 rouge).
- Distribution tubing will show corrosion effects and accumulated rouge (class 2 and 3 rouge).
- Ball valves can generate corrosion from trap lines as well as at use points.
Further, as a function of time, these corrosion factors may produce corrosion products as they meet, combine, and overlap with a blend of ferrous and ferric rouge. Generally, black rouge is first seen in the generator; rouge then emerges at the generator discharge piping and eventually throughout the CS distribution system.
SEM OBSERVATIONS
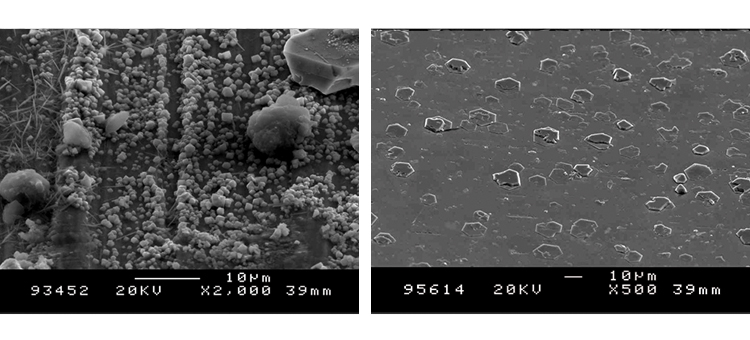
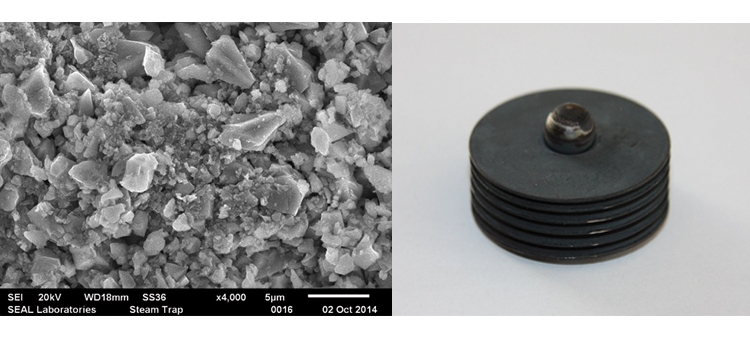
We conducted SEM analyses to illustrate the microstructure of the corrosion byproducts that covered the entire surface with crystalline and other particles. The background or underlying surface upon which the particles are distributed ranged from various gradations of ferrous (Figures 1–3) to the most ubiquitous sample, a silica/ferrous, glassy, tenacious, uniform deposit (Figure 4). A steam trap bellows (Figures 5–6), was also investigated.
AES results
AES testing is an analytical method used to determine the surface chemistry of stainless steel and predict its corrosion resistance. It also shows the degradation of the passive film and the reduction of chromium concentration in the passive film as the surface degrades due to corrosion.
AES survey scans (depth profiles of elemental concentrations at the surface) were used to characterize the elemental composition of each sample surface. The analysis sites and SEM magnifications were carefully selected to provide information from typical regions. Each survey provided information from the top few molecular layers (estimated at 10 ångstroms [Å] per layer) down to the alloy metal depth (200–1,000 Å). Various amounts of iron (Fe), Cr (chromium), oxygen (O), nickel (Ni) and carbon (C) were found in all areas of rouge. AES figures and results are described in the Case Studies section.
Typical AES results of initial conditions show heavy oxidation on the received sample with very high Fe and O concentrations (iron oxide) and low Cr at the surface. This rouge buildup leads to particulate release and potential contamination of product and product-contact surfaces. After the rouge is removed, the “passivated” samples show complete restoration of the passive film, with Cr reaching a higher concentration than Fe, and a Cr:Fe ratio from 1.0 to 2.0 at the surface, with a distinct lack of iron oxides.

XPS results
Some rouged surfaces were analyzed using XPS/ESCA to compare the elemental concentrations and oxidation states of the spectra for Fe, Cr, sulfur (S), phosphorous (P), sodium (Na), calcium (Ca), and nitrogen (N), as well as O and C (Table A). Cr content varies from near-passive-layer values to lower values typically found in the base alloy. The Fe and Cr levels found on the surface are indicative of different thicknesses and classes of rouge deposits. XPS testing reveals increases in C, Na, or Ca in the rouged surfaces over the clean and passivated surface.
| Element | Case 1: Rouged | Case 3: Rouged | Case 3: Passivated |
|---|---|---|---|
| Carbon | 46.0 | 64.2 | 37.1 |
| Chromium | 7.7 | 1.1 | 6.9 |
| Iron | 5.3 | 1.0 | 2.8 |
| Nitrogen | <1.0 | 4.4 | 3.2 |
| Oxygen | 40.5 | 25.2 | 47.9 |
| Phosphorous | 1.1 | <1.0 | <1.0 |
| Sodium | 5.9 | 2.1 | 2.0 |
XPS testing also shows the ferrous (black) rouge contains a high C content as well as Fe(x)O(y) (iron oxides) within the rouge. XPS data was not significantly helpful in understanding the surface changes during the corrosion process, since it evaluates the rouge and the base metal concurrently. Further XPS testing using more samples are required to be able to evaluate the results. Previous authors also had difficulty evaluating XPS data.10
Field observations during actual removal revealed the C content was high and generally removed via filtration during the processing. SEM micrographs taken before and after derouging treatments illustrate the surface damage created by these deposits, including pitting and porosity, which are a direct effect of corrosion.
XPS results after passivation show a much higher Cr:Fe content ratio at the surface as the passive film is reformed, reducing the corrosion rate and damaging effects on the surface.
Chemical processing
Sample coupons showed substantial increases in the Cr:Fe ratios between “as-received” surfaces and passivated surfaces. As-received sample Cr:Fe ratios tested between 0.6 and 1.0, while the passivated after-treatment ratios ranged between 1.0 and 2.5. Typical values for electropolished and passivated stainless steel range between 1.5 and 2.5. The depth of the maximum Cr:Fe ratios (determined by AES) ranged between 3 and 16 Å on the after-treatment samples. These compare favorably to previous research data reported by both Coleman2 and Roll.9
All samples had typical levels of Ni, Fe, Cr, O, and C on the surface. Low levels of P, S, Cl, Ca, N, and Na were also detected on most samples. These are common residues of cleaning chemistries, purified water, or the electropolishing process. In subsequent analyses, a slight Si contamination was detected at differing levels on the surface and on the austenitic crystals themselves. Their source is the silica content of the water/steam, mechanical polishing compounds, or visual sight glasses slowly dissolving or etching within the CS generating unit.
Corrosion Products
Corrosion products encountered in CS systems, as noted, are highly variable. This is due not only to the variety of conditions within these systems, but also the placement of assorted components such as traps, valves, and other appurtenances, which can give rise to corrosive conditions and corrosion products. In addition, replacement components that have not been well passivated are introduced into the system all too often. Corrosion products are also heavily influenced by the design of the CS generator and water quality.
Some generator unit types are reboilers, while others are tube flash evaporators. CS generators usually utilize terminal mesh screens to remove moisture from clean steam, while others employ a baffle or cyclone separator. Some develop an almost uniform ferrous patina within the distribution tubing, accompanied by overlying ferric rouge.
Units with baffles generated not only a dark ferrous film with ferric oxide rouge beneath, but also formed a secondary upper surface phenomenon of a soot-like rouge, which may be more easily wiped from surfaces. In general, this ferrous, soot-like deposit is considerably more pronounced than the ferric rouging and much more mobile.
The rouge that forms in the condensate trail at the bottom of distribution tubing has ferric oxide rouge on top of the ferrous rouge, due to the higher oxidation state of iron in the condensate fluid. The ferric oxide rouge migrates through the condensate traps, is evident in drain lines, and the upper layers are easily wiped from the surface.
Water quality plays a major part in rouge product chemistry. Higher hydrocarbon levels generate additional black carbon in the rouge, and higher silica levels lead to higher silica content, forming a shiny or smoother rouge layer. As mentioned above, water level sight glasses have also been shown to erode, releasing their silica and debris into the system.
CS Systems Case Studies
Rouge is a concern in steam systems because it forms relatively thick layers that generate particles. These particles can be found on steamed surfaces or in steam sterilization equipment. The potential effect on pharmaceutical products is presented in the following sections.
Case 1
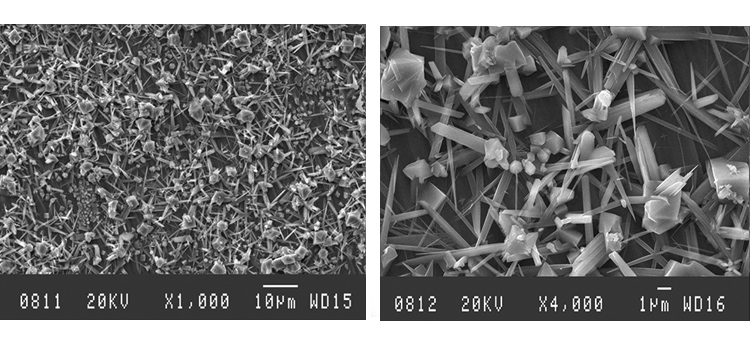
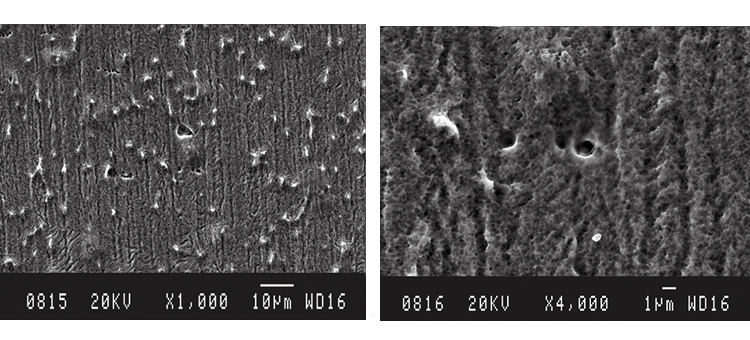
The as-received sample SEMs shown in Figures 7 and 8 illustrate the microcrystalline nature of the class 2 rouge in Case 1. The iron oxide crystals form a relatively tight matrix on the surface, appearing like a fine-grain residue. The derouged and passivated surface shows the damage caused by corrosion, producing the rough and slightly porous surface texture shown in Figures 9 and 10.
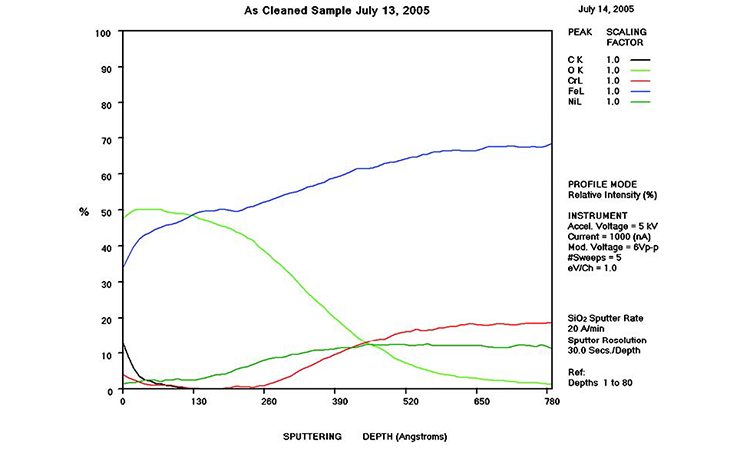
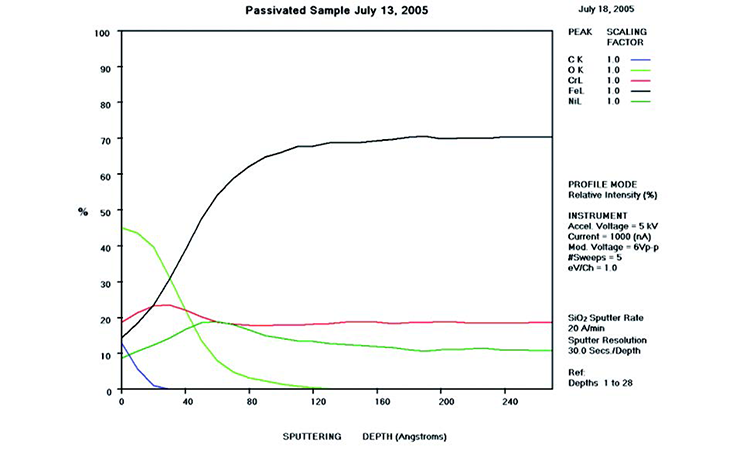
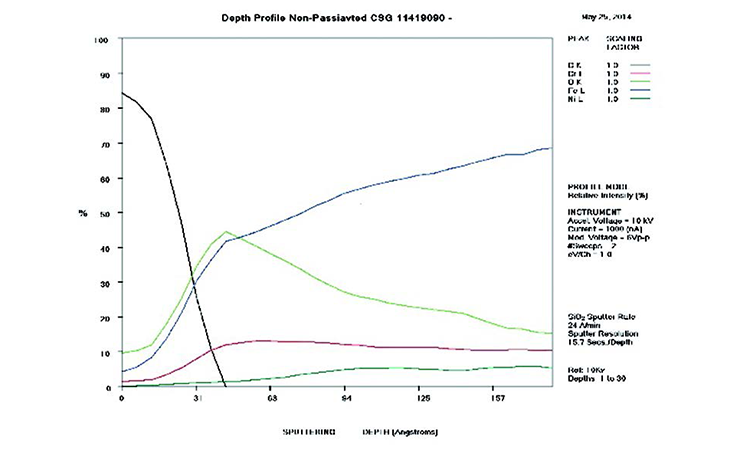
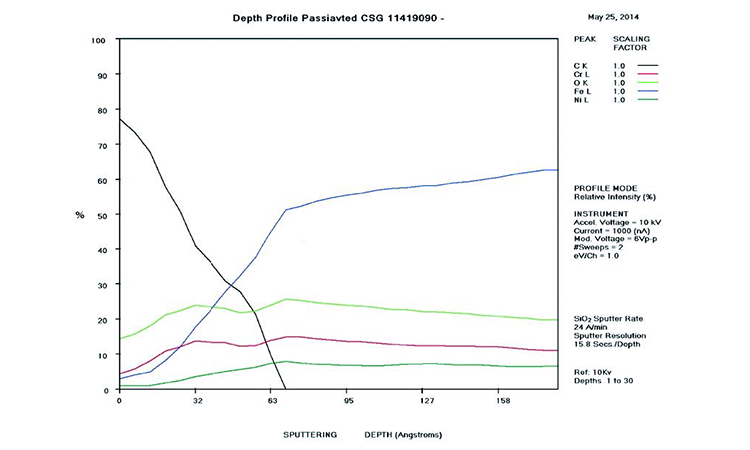
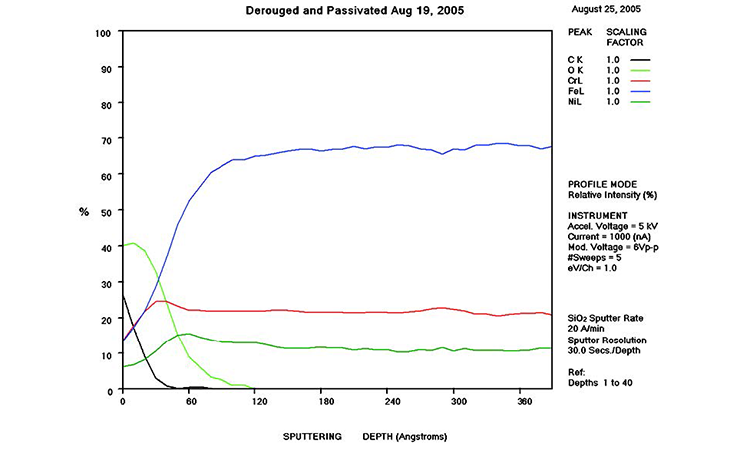
The AES scans in Figure 11 show the original surface condition in the as-received sample with heavy iron oxide on the surface. The derouged and passivated surface (Figure 12) shows that the passive film has attained a slightly higher Cr (red line) content above the Fe (black line) at > 1.0 Cr:Fe ratio. The thin (< 80 Å) chromium oxide passive film is much more protective than the hundreds of ångstroms thick iron oxide crystalline film of the rouge layer and base metal, with over 65% Fe content. The chemistry of the derouged and passivated surface is now similar to a passivated mill-finished material.
The rouge in Case 1 is a class 2 rouge that formed in place; as the rouge accumulates, this type produces particulates that grow in size and migrate with the steam. The corrosion exhibited in this case does not severely pit or significantly degrade the surface. Derouging on a regular basis will limit both corrosive effects on the surface and remove potential for excessive migration of particles that often reach visible size range in fluids or products.
In Figure 11, AES results show a thick layer high in Fe and O (500 Å iron oxides; blue and lime green lines, respectively) near the surface that transitions to alloy levels of Fe, Cr, Ni, and O. The Fe concentration (blue line) is significantly higher than any of the other metals, rising from 35% at the surface to over 65% in the alloy. The O level (lime green line) transitions from nearly 50% at the surface to near zero in the alloy at more than 700 Å in depth of the oxide film. The Ni (dark green line) and Cr (red line) levels start very low at the surface (< 4%) and transition to normal levels (11% and 17%, respectively) at alloy depth.
The AES chart in Figure 12 shows that the rouge layer (iron oxides) has been removed and the passive film has reformed. In the first 15 Å, the Cr level (red line) is above the Fe level (black line), indicative of the passive film. The Ni level starts at 9% on the surface and rises to above the Cr level (± 16%) between 60 and 70 Å, then transitions to the alloy levels at 200 Å. The carbon level (blue line) starts at 12% and drops to zero at 30 Å. The Fe level starts low (< 15%) and rises to equal the Cr level at 15 Å and continues to the alloy level of over 65% at 150 Å. The Cr level rises from the surface to a level of 25% at 30 Å, returning to 17% in the alloy. The high O level near the surface (lime green line) drops to zero after a depth of 120 Å. This analysis shows a well-developed passive film on the surface.
The SEM photos in Figures 13 and 14 illustrate the rough, rouged, and porous crystalline nature of the class 1 and 2 ferrous oxide layer on the surface. The derouged surface shows the effects of the corrosion in its roughened, partially pitted surface (Figures 18–19).
Case 2
As noted, the derouged and passivated surface in Figures 13 and 14 lost its heavy oxidation. Figures 15 and 16 show a restored passive film on the metal surface. In Figure 17, the Fe concentration (black line) drops dramatically throughout the near-surface area (< 100 Å), while the Cr content (red line) increases in the first 20 Å to develop a passive film, with a Cr:Fe ratio of approximately 1.5 in the first 25 Å of the surface (approximately three molecular layers). The O level (lime green line) remains high in the first 30 Å of the passive film. The C level (blue line) remains high at the surface, but drops as the surface transitions into the alloy.
Case 3
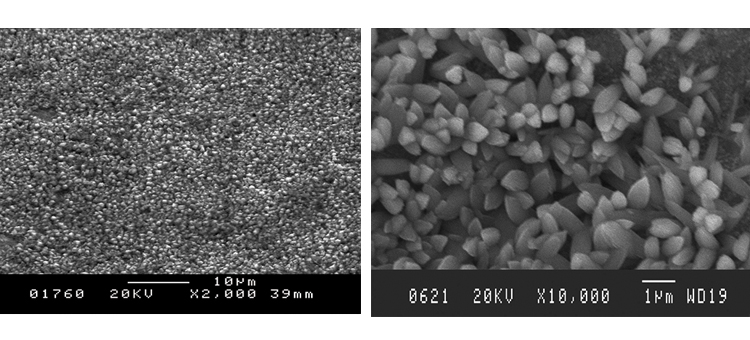
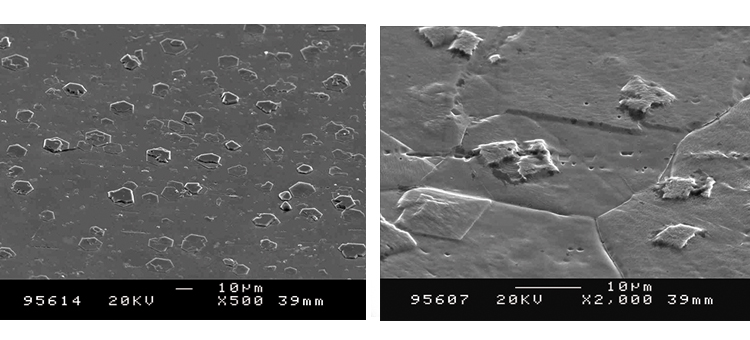
Figures 18 and 19 illustrate the size of oxide crystals that can grow from steam corrosion. These class 2 corrosion products begin as very small crystal growths on the surface, then grow into particles from 5 to 50 micrometers (μm) in size and even larger. These crystals may be released into the steam and migrate into the process stream or onto product contact surfaces.
Figures 20 and 21 show conditions of the CS tubing in the distribution system.
Figures 22 and 23 show that the clean derouged surface is void of the iron oxide material, with minor pitting and roughness generated from the corrosion of the surface. The SEMs show the surface rouge as inspected. The ferrous oxide rouge on the surface has a thin nonuniform overlaying film of ferric oxide rouge.
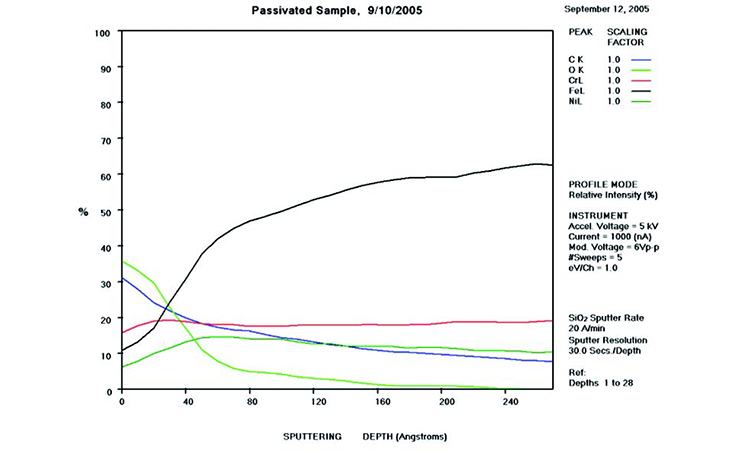
The AES scan of Figure 24 shows excessive carbon and iron oxide on the rouged surface with no passive film. Figure 25 shows the surface after derouging and passivation, with recreation of the passive film and loss of the iron oxide film. In the first 50 Å of the as-received sample, the C, O, and Fe levels are very high, showing the iron oxide film with elemental carbon on the surface, typical of CS rouge of class 2 corrosion with a high carbon level.
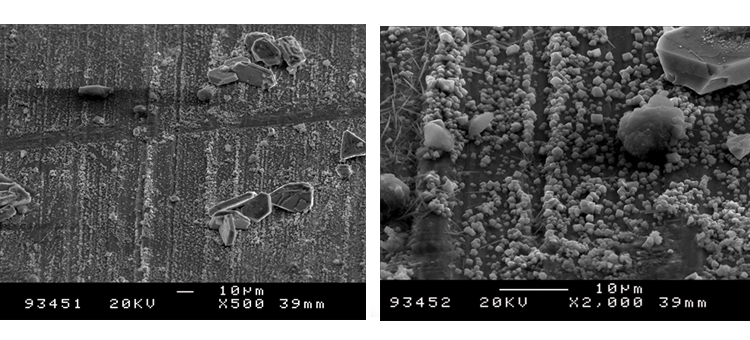
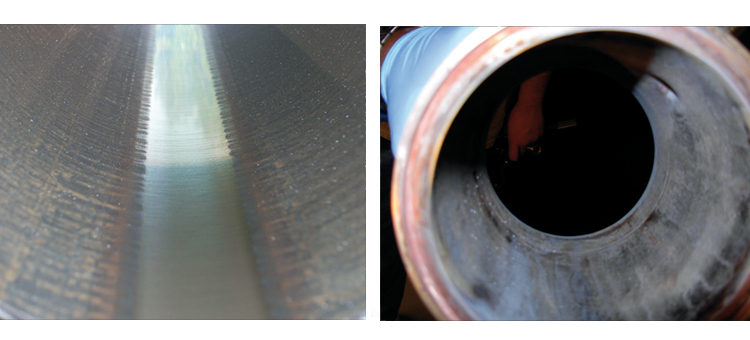
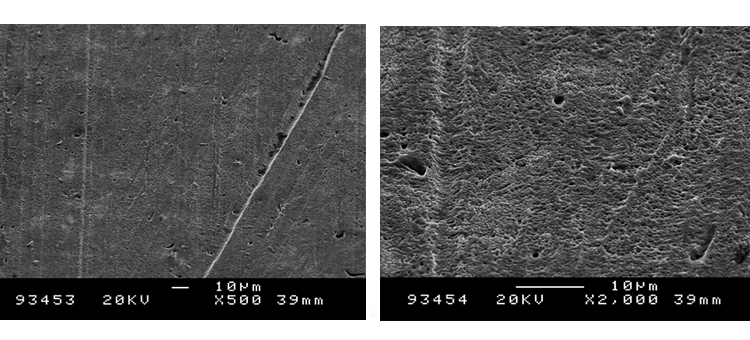
Case 4
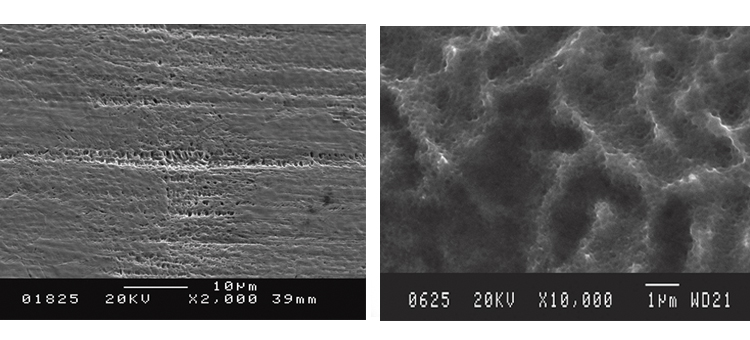
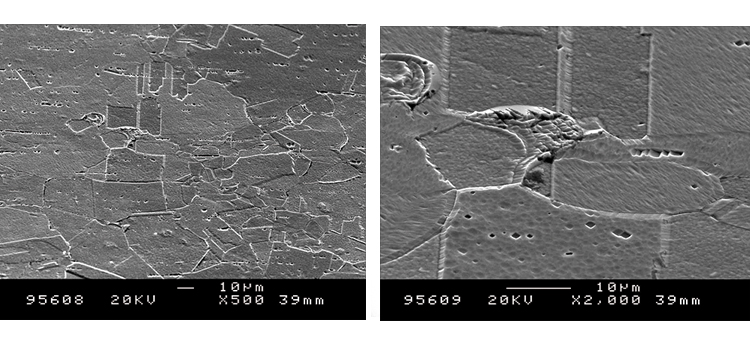
Figures 26–29 show how a shiny black surface appears microscopically. The surface is much smoother than typical rouge crystals due to the amorphous silica that appears like a glassy coating. Once removed, however, the surface reveals its low-level pitting and austenitic metallic crystal edge deformation.
Figure 30 reveals establishment of the passive film after iron oxide deposits were removed. The Cr:Fe ratio at the surface is slightly greater than 1:1 in the first 20+ Å, as the Fe (blue line) and Cr (red line) merge toward the surface. The O level (lime green line) starts high at the surface (at 40%) and drops to zero at 120 Å, while the Ni level (dark green line) begins at 7%, rises quickly to nearly 15%, then then levels off at about 10% into the alloy composition below 150 Å.
Measuring soluble metals and particulates
CS systems can be monitored for metals in the condensate and steam flow, measuring the number and size of particles from 5 to > 100 μm. The results presented in Table B show ranges of metal content and particulate in the CS critical utility of three case studies. Particle sizes above 50 μm are visible contaminants,3 and significant numbers of particles greater than 50–100 μm present a high risk for contamination on surfaces that are steamed by this critical utility.
| Size, μm | > 5 µm | > 15 µm | > 25 µm | > 50 µm | > 100 µm |
|---|---|---|---|---|---|
| Size, μm | > 5 µm | > 15 µm | > 25 µm | > 50 µm | > 100 µm |
| Sample | Particle count per 100 milliliters condensate | ||||
| CSG 1 | 166 | 200 | 133 | 30 | 1 |
| CSG 2 | 310 | 84 | 56 | 22 | 2 |
| CSG 3 | 5330 | 6400 | 4660 | 166 | 18 |
| CSD 1 | 200 | 45 | 21 | 34 | 1 |
| CSD 2 | 404 | 81 | 26 | 4 | 0 |
| CSD 3 | 2020 | 403 | 132 | 22 | 8 |
CSG: Clean steam generator CSD: Clean steam distribution
Removing corrosion byproducts
Ferrous oxide rouge deposits may be removed using organic acids with chelant combinations (and other variable complexes) in the proper concentrations, contact times, and temperatures. Other advocated mineral acid treatment approaches include, but are not limited to:
- Commercial acid detergents
- Mineral acids with halogenated additives, such as ammonium bifluoride
- Phosphoric acid blends
- Various chemical pickling remedies
The objective of the derouging process is to remove the iron oxide deposits while protecting the stainless steel substrate surface from any additional pitting corrosion.
To ensure that polished surface finishes are not damaged by the derouging solutions, it’s also important to avoid aggressive techniques that can remove base metal. Following the rouge and oxide deposit removal, a passivation treatment can restore the passive film by removing elemental iron and iron oxides from the first few molecular layers in the surface while maintaining the protective chromium oxide layer. This can minimize continued corrosive mechanisms upon return to service.
Conclusion
The corrosion byproducts encountered in clean and pure steam systems—carbon, silica, and iron oxide compounds—are present to some degree in every system. Many CS systems lack proper routine inspection and maintenance that could control corrosion and particulate migration from oxide deposit exfoliation.2 Corrosion problems are exacerbated by poor gasket specifications, components with dissimilar metals, and decreased stainless steel surface quality, as well as the uncontrolled nature of mechanical/electrochemical polishing materials and methods combined with poor material handling and lack of routine derouging and passivation techniques.7
It has also long been suspected that stainless steel materials are not necessarily delivered at a desirable quality level. Manufacturing processes, combined with subsequent material handling unit operations and fabrication techniques, establish the surface chemistry (chromium oxide content of the passive film), corrosion resistance, and surface finish quality, which all affect the final product.
Claims that these grayish/black deposits are stable, inevitable, and should be left alone have very little credibility. Corrosion produces rouge that is evidenced as discolored stains on product contact surfaces, and generates mobile particles that accumulate on steam sterilized surfaces. CS rouge contaminants have been found in final filtration processes, becoming a potentially uncontrolled material in the final process fluids and gasses. While we acknowledge that the examples presented here are specific cases, they are not unique. They are similar to cases found within other systems, especially those where corrosion has been left to continue without proper corrective treatment.4
Finally, corrosion within CS systems will generate migratory rouge that can be identified and measured in the steam, the condensate, and on the system interior surfaces. Proper design and maintenance is critical in the operation of CS generation and distribution systems for high-purity applications.
Future research measuring the time, conditions, and properties of rouge development could be compared to particulate generation to establish risk of product contamination. Systematic, routine measurements over a two-year period could track corrosion products to illustrate changes in particulate release and transport within the CS system studied, as well as the surface conditions within the generation and distribution equipment.
Acknowledgments
The authors would like to give credit (posthumously) to Robert W. Evans for his efforts in joining Drew Coleman during initial research and drafting phase of this article. He envisioned the need to bring to the industry the effects of corrosion and subsequent rouge products in CS systems with their potential for detrimental process or product contamination. In addition, we would like to thank Dr. Brent Ekstrand for his editing efforts in finalizing this technical article to more clearly bring our message to the industry.
References
- 1American Society of Mechanical Engineers. ASME BPE Bioprocessing Equipment Standard. 2014.
- 2
- 3Essmann, M., and R. Gomez. “Quality Requirements for Pure Steam.” In Pharmaceutical Water. GMP Publishing, 2012. https://www.gmp-publishing.com/media/files/table_of_contents/Pharmaceutical_Water_TOC_web.pdf
- 4Evans, R.W., and D. C. Coleman. “Corrosion Products in Pharmaceutical/Biotech Sanitary Water Systems.” Parts 1 and 2. Ultrapure Water 16, nos. 8 and 10, October 1999.
- 6International Society for Pharmaceutical Engineering. Critical Utilities D/A/CH COP. “Rouge in Pharmaceutical Water and Steam Systems, Pharmaceutical Engineering 29, no. 4 (July-August 2009).
- 7———. Water and Steam Systems: Pharmaceutical Engineering Guides for New and Renovated Facilities. Baseline Pharmaceutical Engineering Guide, Volume 4, first edition. 2001.
- 9Roll, D., and R. Webb. “Developing an Effective Passivation Process to Maintain Laser Mark Integrity for Medical Device Components.” Medical Product Outsourcing, 9 December 2010. http://www.mpo-mag.com/contents/view_technical-features/2010-12-09/developing-an-effective-passivation-process-to-mai
- 10Tverberg, John C., and James A. Ledden. “Rouging of Stainless Steel in WFI and High Purity Water Systems,” Presented at Institute for International Research, “Preparing for Changing Paradigms in High Purity Water,” San Francisco, California, October 1999. http://www.csidesigns.com/uploads/white-papers/rouging.pdf


