2020 ISPE Pharma 4.0™ Virtual Conference Highlights

Through this difficult time of the COVID-19 pandemic, ISPE has remained active. At the 2020 ISPE Pharma 4.0™ Virtual Conference, 17–18 November, 174 attendees gathered online to discuss and learn about the progress of the pharma-specific industry 4.0 approach, Pharma 4.0™ (now a registered trademark in the European Union).
This article covers some highlights of the conference.
Amit Nastik, Novartis

Amit Nastik, Global Head Strategy & Operations and Local Markets Manufacturing, Novartis, opened the conference with the first presentation on his vision of Pharma 4.0™ operations. He categorized the opportunities to embrace data and digital across the pharma value chain in three areas.
- Innovation:
- Bring clinical trials to the patients.
- Combine medicines with cutting-edge technology.
- Mine clinical trial data for new insights.
- Operations:
- Streamline scheduling and shop floor control using integrated manufacturing execution systems and enterprise resource planning.
- Automate and replace manual operations through robotics.
- Use artificial intelligence (AI) to enable demand forecasting and integrate sales and operations.
- Engagement:
- Transform pharma commercial models.
- Launch excellence along the patient funnel (i.e., the customer’s path from brand awareness to brand loyalty and advocacy).
- Provide innovative digital solutions for customers.
Although there are these opportunities, Nastik noted that the pharma industry tends to be cautious about embracing new technologies for a variety of reasons: Pharma is a highly regulated industry where every change in manufacturing needs to be registered and documented. Pharma is historically a very strong, high-margin business with the focus more on developing new products than on process improvements. Additionally, top-line growth, quality, and product availability are main priorities. Hence, pharma manufacturing is much less advanced than manufacturing in other industries with regard to continuous process flow, extensive automation, supplier integration, and continuous in-process control and monitoring.
Nastik explained that applying Pharma 4.0™ levers will lead to efficiency gains across various dimensions. Advanced analytics and machine learning (ML) will support cognitive processes and require decision-making based on data and analysis. Robotic process automation will be applied to processes with a large share of manual and repetitive tasks, and physical robots will perform processes to move physical goods. All of these uses of technology will result in better buying decisions and improved flow of materials, integrated and digitalized business planning, connected sites making medicines efficiently, streamlined and faster batch release, end-to-end product traceability, and an uninterrupted supply of high-quality medicines to patients.
Teresa Rodo, Merck Healthcare KGaA
Teresa Rodo, Executive Vice President Global Healthcare, Merck Healthcare KGaA, spoke on the theme “boost and sustain performance” and emphasized that the principle of “from purpose to people through performance” can help the industry successfully realize the power of digitalization.
To illustrate the “power of purpose,” she recalled John F. Kennedy and his mission to bring a man to the moon in the 1960s. Even the cleaning service personnel at NASA’s Cape Canaveral facilities understood their work as a part of this mission.
At Merck, the purpose is dedication to human progress—this is the leading idea guiding the healthcare strategy for the company’s products.
Rodo said that being a global innovator starts with shop floor employees, who are now working with real-time performance-indicator dashboards (no more paper).
She also noted the role of operations in providing a competitive advantage for Merck. Two of the main drivers in operations are cost and cash competitiveness and supply flexibility. Merck defines their operational levers, midterm goals, and yearly objectives with great visibility and transparency—which is only possible when operations are supported by digitalization.
Rodo said that the engagement of people is of equal importance in operational success; this is the emotional aspect of the business, with a focus on mindsets and behaviors. Diversity and inclusion are essential, and they are being achieved through communities of volunteers, training, the ISPE Women in Pharma® program, objectives for senior leaders, and other initiatives. Another driver of engagement is sustainability. For example, “green” teams are prioritizing efforts to lessen the use of plastic materials to protect the environment.
As a final point, Rodo said that using all forms of modern media not only engages people and generates momentum but also helps organize interactions among all stakeholders in the organization. It is important to reward success and recognize people’s performance and resilience in this complex new world of digitalization.
James Thomas, Bayer AG
James Thomas, Head of Digital Strategy, Bayer AG Pharmaceutical Division, gave a talk titled “Driving Digitalization for Pharma Manufacturing.”
He identified several opportunities associated with using digitalization to connect key equipment and lines across the manufacturing network: get-ting real-time operational data, deploying advanced analytics, data sharing to enhance product launches and predictions, and avoiding machine down-time and equipment failure. As challenges, he noted a historically heterogeneous equipment landscape, the current management of data/buffering, the data integration blueprint, and the focus on brownfield and greenfield sites.
Regarding digitalization of the end-to-end supply chain, Thomas envisions the achievement of autonomous planning and identified as opportunities a new level of transparency that allows companies to do simulations; the integration of suppliers, raw materials, and contractor‘s activities; and the better identification of downstream costs and opportunities. As challenges, he identified multiple internal and external data sources; the so-called last mile for data (i.e., the telecommunications networks that deliver data to end users), which often has limitations or is costly to manage; the limited willingness of many to share data; and the need to take a leap of faith in change management.
In practical terms, Bayer combines data in a data and analytics platform to implement use cases, drive collaboration, and create new business models in lighthouse projects. Another key success factor is developing skills and a “digital mindset” across the organization.
It is important to reward success and recognize people’s performance and resilience in this complex new world of digitalization.
Dave S. Sternasty, Eli Lilly and Company
Dave S. Sternasty, Vice President, Corporate Engineering and Global Health, Safety, and Environment, Eli Lilly and Company, shared his perspective on future operations in an era of technology and digitalization.
He said the three integrated priorities of manufacturing are supply, launch, and productivity. The digital plant agenda is built on a strong foundation with the steps being:
- Pre-digital plant
- Digital silos
- Connected manufacturing (the situation today)
- Predictive plant (the company’s goal for 2023)
- Adaptive plant
The elements to achieve a predictive plant are in six areas: data and analytics, supply chain, laboratories and quality, manufacturing execution and automation, manufacturing support, and facilities and security/integration.
Sternasty used a parenteral filling line as a real-time predictive analytics example. A decontamination process with a four- to six-hour cycle time and a certain variability in performance could be managed to predict the expected outcome within four minutes and with 87% accuracy. This would help increase line availability and productivity and would improve process knowledge.
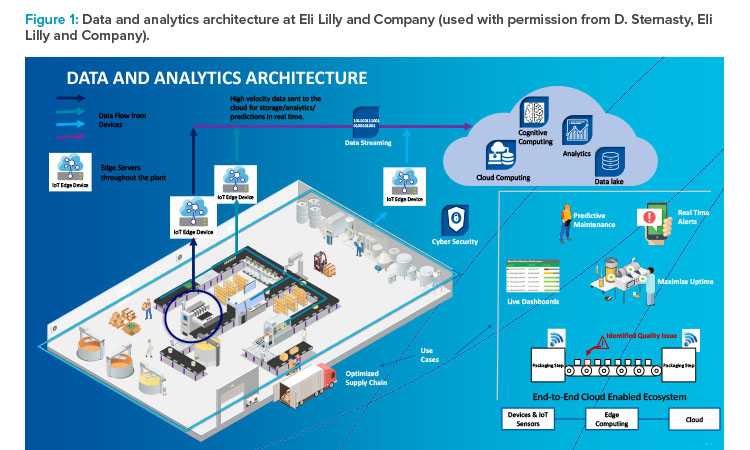
Figure 1 represents Eli Lilly’s data and analytics architecture, which starts with internet of things (IoT) devices that collect data from the shop floor and ends with information stored in the cloud. It is an end-to-end connected system that gives information about real-time events, issues, and production progress to decision-makers. Even the predictive maintenance program is integrated.
Giuseppe Recchia, Davinci Digital Therapeutics
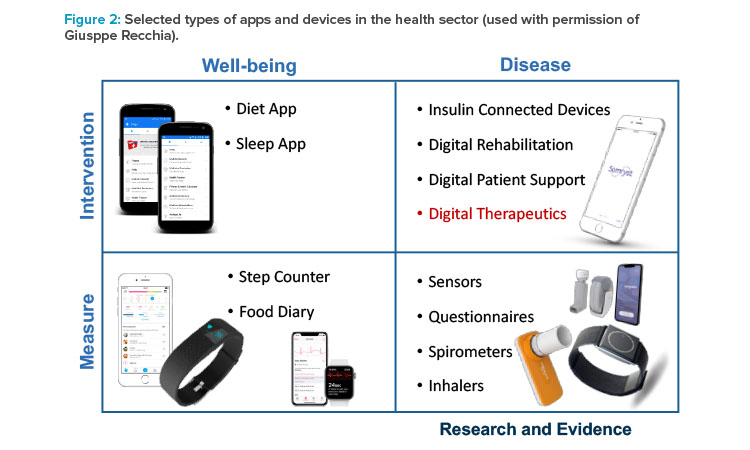
Giuseppe Recchia, Cofounder and CEO of DaVinci Digital Therapeutics, Milan, Italy, presented on digital health approaches. He noted that digital health tools have a range of purposes and uses, from promoting well-being by allowing consumers to track their diets, sleep, and activity to helping patients and providers manage serious diseases and providing data for research (Figure 2).
Digital therapeutics is a subgroup of digital health with well-defined purposes: to treat and manage specific diseases or conditions. Because they are designed to modify disease states, digital therapeutics require regulatory agency approval and a physician prescription, and the cost may be covered by health insurance. As examples of successful digital therapeutics applications, Recchia noted those used in the surveillance of oncology therapy and for treatment of insomnia.
He explained that the “active ingredient” in digital therapeutics is an algorithm. Digital “excipients” include reminders, appointment managers, scheduling tools, automated assistants, tools to alert or contact physicians, patient education, data integration, gamification, media libraries, progress tracking tools, rewards to reinforce patient behaviors, feedback, and social media applications to provide peer support and comparisons.
Like drugs, digital therapeutics are developed in a preclinical phase and a clinical phase. Digital therapeutics development is a structured process with research, software devlopment, pilot clinical development, and full clinical development including randomized controlled trials. After that, there is a submission-approval process followed by a postmarketing surveillance.
Recchia also noted some issues with digital therapeutics. These include creating a level of evidence to demonstrate clinically relevant results, reimbursement challenges, and questions about the design of clinical trials, such as how to test digital therapeutics in an “analogical” trials environment.
Rechhia emphasized that in chronic desease management, digital therapeutics normally will not substitute for a drug therapy; instead, they work as additive therapies, which can improve patient outcomes, increase the therapeutic value of a drug, optimize a drug life cycle, create access to real-time data, personalize a drug therapy, and thus round out the therapeutic offering.
Barry O’Sullivan, University College, Cork, Ireland
Barry O’Sullivan, Professor, School of Computer Science and IT, University College Cork, Ireland, gave a presentation titled “Artificial Intelligence for Connected Health Post COVID: Technical and Ethical Challenges.”
Noting that the term “artificial intelligence” (AI) was invented in 1955, when people still thought that computers could “think,” O’Sullivan explained that it took a long time to develop the first AI technologies (e.g., game computers, self-driving cars, and robots). Now AI has advanced to the point that retail companies can use data analytics to predict customer behaviors.
The use of AI has ethical implications; for example, AI might be used to influence outcomes of democratic elections. O’Sullivan emphasized that AI methods themselves are not the problem—it is the way we use them.
How are AI and ML helping to fight COVID-19? As an example of an established AI application, O’Sullivan noted the use of computed tomography im-ages to diagnose COVID-19. Apps can also organize and provide access to information about the development of infections. A Canadian start-up company has used AI to anticipate outbreaks, mitigate risk, and build resilience. Another startup, Closed Loop, uses AI to prioritize resources for those most vulnerable to COVID-19 complications. Huge volumes of data from public sources are available, and AI gives scientists ML-enabled capabilities to search the COVID-19 data set. Researchers from Harvard University and the Massachusetts Institute of Technology are currently developing a COVID-19 AI diagnosis application using only coughs recorded from phone calls.
Turning to the regulatory framework, O’Sullivan mentioned the European Commission’s “Coordinated Plan on Artificial Intelligence,”1 published in 2018. Two of the main outcomes of the related High-Level Expert Group on Artificial Intelligence are the “Ethics Guidelines for Trustworthy AI,”2 and the “Policy and Investment Recommendations for Trustworthy AI.”3
The following requirements for AI are outlined in the Ethics Guidelines:
- Human autonomy and agency
- Technically robust and safe
- Privacy and data governance
- Transparency
- Diversity, nondiscrimination, and fairness
- Societal and environmental well-being
- Accountability
An assessment list is available to check any system against these requirements. The next step for the expert group is a white paper on risk-based AI regulation. Possible requirements could address training data, data and record keeping, information to be provided, robustness and accuracy, human oversight, and specific requirements for biometric identification.
Panel Discussion
After their individual presentations, all of the speakers participated in a panel discussion moderated by Chloe Lang, Manager of Data Science EMEA, Sartorius, and ISPE Emerging Leader, and Christian Wölbeling, Executive Industry Advisor, Körber Pharma Software, and founder of the ISPE Pharma 4.0™ Special Interest Group (SIG). The key questions addressed were:
- What is the biggest hurdle for Pharma 4.0™ digitalization?
- Who is driving the change: which department or organization?
- How do universities play a role? What collaborations are happening?
Overall themes that emerged throughout the discussion were embracing new talent and skills, creating an environment for “out of the box” thinking, collaboration, and integration.
The main rationales for moving toward Pharma 4.0™ are a combination of strategy, business cases, and problem-solving. Finding problems to solve helps make digital changes tangible and is a way to attract new talents, develop new skills, and accelerate entrepreneurial thinking, which helps companies and the workforce see and embrace potential changes. Collaboration both internally within companies and externally with universities and suppliers is crucial to drive a Pharma 4.0™ vision.
Panelists highlighted that these changes cannot be managed by one function within an organization; there has to be a multifunctional approach. For example, IT can enable the changes, but business and strategic objectives need to be considered. In many cases, companies have established partnerships that have been in place for many years. But understanding how universities are fostering digital aptitude in their graduates is also important for working with and attracting the younger workforce. Overall, more engagements are starting in areas like smart manufacturing initiatives to move AI and ML to manufacturing environments. As more data become available, more opportunities arise, which also brings the challenge of working out where to start and how to identify what is significant and interesting. With all the focus on AI and ML, it is also important to remember that implementing algorithms is not the only challenge—stakeholders must also consider how to interpret and use the data in a validated environment and how to manage the life cycle efficiently.
Although the concept “digital transformation” may be easy to define, it can be difficult to ensure everyone has the same understanding and expectations. Panelists said that integration remains the biggest hurdle. In addition to data and interface integration challenges, integration will involve breaking down cultural silos and filling the need for a workforce with cross-functional skills. Product integration is important both to ensure patients have the ability to use digital therapeutics products, and to ensure data and information flow automatically and efficiently within organizations.
By creating an environment where not only the data but also people and functions are interconnected, organizations can foster space for innovation, try different approaches, and take radical steps. The integration of industry, suppliers, and contract manufacturers also helps build relationships and understanding, creating more of a community and network. For example, while it is not possible to visit contract manufacturers at the moment, having systems set up to share data and information makes it easy to follow what is happening at production sites without having to visit in person. In digital therapeutics and other areas of digital innovation, the need for rules, guidance, and collaboration across industry and regulatory/government agencies was also highlighted.
One of the closing thoughts was that transformation is a marathon, and it takes time and clear change management to ensure transformations are impactful; if the organization is not engaged, the benefit of digitalization will be low.
Heike Roeder, Bayer Germany
Heike Roeder, Lead Digital Transformation QMS, Bayer Germany, presented on “My Life in Quality 4.0.” She explained that a quality manager has a legal responsibility for quality oversight of operations, quality, and compliance. This responsibility will not change in a digitalized world, but the methods to achieve it will. She described the changing decision-making paradigm, which is impacted by the connectivity of data and people. This already requires a cultural change toward openness and transparency, and away from functional silos and organizational chart–defined responsibilities. Data ownership means responsibility for the correctness of data and data integrity—it is not about “possession” of data interpretations of sovereignty over the “ownership” of data.
Content-wise, the paradigm change goes from retrospective control to predictive prevention. Also, the paradigm should be patient-centric: i.e., the patient outcome will be more in focus than in the past, when comparisons to defined and approved specifications were the only criteria for quality.
Roeder next presented a practical business case driven by the Pharma 4.0™ approach: the electronic standard operating procedure (SOP). The SOP of the future is digital, user-friendly, precise, and intuitively easy to understand. It is mobily accessible from everywhere, provides the right information at the right time and the right place, and is delivered in a customized, attractive format (which might include text, voice, graphics, or video).
Alexandra Grebe De Barron, Bayer AG
Alexandra Grebe de Barron, Data Product Owner in Digital Transformation & IT, Bayer AG, presented “FAIR data for Better Patient Outcomes.” FAIR data and digital assets are findable, accessible, interoperable, and reusable. This concept clarifies the context, meaning, trustworthiness, and origin of data, and how data can be used, with a clear and accessible data usage license.
Grebe de Barron said data-driven insights are redefining the health services landscape: Patients, payers, and providers are becoming “super consumers” empowered by data. “Sick care” is turning into healthcare focused on prevention and the affordability of care. Blockbuster drugs will increasingly be replaced by precision medicine that uses data mining to deliver therapies tailored to specific patients. Disconnected healthcare will become connected healthcare as systems integrate healthcare data, human efforts, and machinery to offer better interventions.
Algorithmic medical applications can identify individual patient risks for a defined endpoint, which helps physicians make the right treatment choices. Based on a new and complete data set, algorithms can be developed to predict the future development of a disease on a patient level.
Philip M. Gammell, Astellas Pharma, INC.
Philip M. Gammell, Associate Director Engineering, Astellas Pharma, Inc., presented a Pharma 4.0™ business case on the “Connected Worker.” The pilot project involved a tech transfer from Astellas to a contract manufacturing organization (CMO) where workers were connected with wearable devices, smart glasses, and mobile devices. The overall approach involved:
- A scope review with the CMO to identify use cases, coordination agreements, and stakeholders
- Infrastructure tests with initial device and platform testing
- Training and live testing before production, with key users, to finalize a testing protocol
- Live testing during production, including creating user credentials, developing procedures, and collecting feedback
- Conducting final use cases at local sites and collecting feedback
- Next steps, such as a road map for implementation with all CMOs
The connected worker pilot met its goal for replacing paper-based processes with digital processes through an innovative solution using augmented reality, smart glasses, and voice-activated and hands-free methods. Additionally, real-time data and key performance indicators are available.
Markus Zeitz, Novartis
Markus Zeitz, Quality Innovation Hub Lead, Novartis, presented on Novartis’s efforts to achieve scalable, centralized, and system-wide audit trail review and visualization. He began with the US FDA definition of an audit trail4—“a secure, computer-generated, time-stamped electronic record that allows for reconstruction of the course of events relating to the creation, modification, or deletion of an electronic record”—and noted that regulations such as Annex 11 and 21 CFR 211 require review of audit trails for GMP-relevant changes and deletions. Audit trail review, he emphasized, is a key activity to ensure data integrity.
Zeitz said that the vision is to change audit trail review from a human-driven, time-consuming, and paper-based process to a full data- and computer-driven process covering metrics, logs, and events. The core element in the Novartis case is a big data analytics platform with online reports, web access, and alerts.
The main process steps are investigating, monitoring, and analyzing data. The consolidation of data after sourcing from various sources is done by robotic process automation. A challenge is the standardization of data requirements across the existing landscape of data sources.
Panel Discussion
A Q&A session with Roeder, Grebe de Barron, Gammell, and Zeitz provided an opportunity for audience members to pose their questions. The main directions of questions may be summarized as follows:
- Challenges to overcome in Pharma 4.0™ implementation, whether detected from the Pharma 4.0™ survey or experienced in projects (including the presented cases)
- Relationships between the Pharma 4.0™ approach and the aims, methods, and practices found in regulations and guidelines (such as the ICH Q series of quality guidelines)
- The role of enabling technologies in designing a Pharma 4.0™ plan of interventions
The holistic approach to Pharma 4.0™ is crucial to overcome the main implementation challenges. Whereas Pharma 4.0™ projects are often seen as technological projects, thinking outside of silos is always key—it is necessary to widen plans, include competencies, involve the organization, and have strong sponsorship. In other words, success depends on approaches and governance that are cross-disciplinary. If this cross-disciplinary need is underestimated, the program is at risk for failure.
For regulations and guidelines, the answers shared in the session may be summarized by the initial statement of the Pharma 4.0™ SIG’s mission: “Provide practical guidance, embedding regulatory best practices, to accelerate Pharma 4.0™ transformations.” The ICH Q series of guidelines, in particular, are an integral part of the Pharma 4.0™ view and method. Every contribution from industry, from authorities, and from the academy is totally welcome and expected.
Regarding the role of enabling technologies in the pharma industry, and others, it is widely observed that a certain effort must be devoted to solving the risk of possible ambiguities in defining such technologies to improve mutual comprehension and create an effective business case. This is an area for the work to come from the Pharma 4.0™ SIG.
The Q&A session also raised matters to be considered for the next editions of the Pharma 4.0™ survey. Pharma 4.0™ approaches help improve the GMP situation, but they have an impact beyond GMP on making processes faster, less costly, and more reliable.














