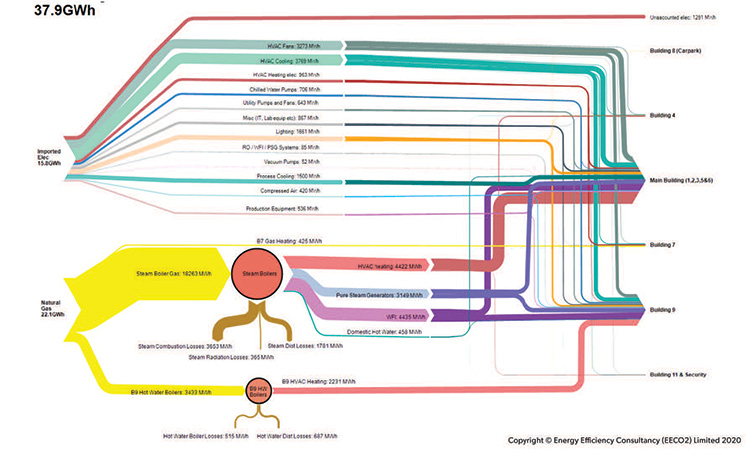
The continuous greening (decarbonization) of electrical grids is already changing our perception of what our priorities should be as we plot the roadmap to zero carbon. For example, in many locations, the use of cogeneration, which was once seen as a major carbon-reduction strategy, can now be defined as having zero or negative carbon benefit, depending on the location and accounting methodology used.
The challenge of setting priorities is particularly evident if we consider the purchase of “green electricity” contracts as an effective carbon-reduction strategy resulting in zero-emission grid power. We know that in a future where we have a 100% renewable grid, the use of (for example) electric vehicles and heat pumps will be genuinely green. But is now the right time to design all of our facilities for this outcome, especially if relatively cheap fuel prices today drive the economics in a different direction? Or does the adoption of these technologies and the additional electrical demand these will make on the system, move the end point further away? To this end, some recent definitions of net-zero carbon propose limiting the use of offsets to only fully optimized energy users.
Industry Innovations
Examples of energy-efficiency success stories can be found throughout our industry. One pharmaceutical company has adopted heat recovery from chillers (a subset of “heat pumps”) as a standard for all new designs. This has improved vendor offerings and designer understanding in this technology, while benefiting those who have not yet tested this approach.
A ground source heat pump provides most of the heating and cooling requirement for a modern lab facility in Freiburg, Germany. Integrated into the building design are very small temperature differences (dTs) for heating and cooling circuits with state-of-the-art fume hood concepts.
These innovations are possible with standard technology available today. We can specify our HVAC to be prepared for low-dT retrofit in future, even if we continue to use steam to temper ambient air in the meantime. A clear priority is to reduce energy waste and inefficiency in our core pharmaceutical processes; otherwise, our utility plant requirements are unnecessarily larger and more expensive to optimize later.
Engineering Challenges
As we undertake this “decarbonization” process of designing for a future with a “100% green grid,” the lack of clear timetables for the various breakpoints challenges all pharmaceutical engineers. Even when we achieve a green grid, there will be no “magic bullet” for zero carbon because pharma companies will remain significant users of heat and other energy-intensive products. Process intensification, continuous processing, and modular containment technologies promise to deliver future facilities that will consume less energy but remain complex. Vendors supplying equipment for these facilities need clear guidance (user requirement specifications) on the specific needs of the facility to achieve optimized equipment designs—and providing this specification requires a strategic vision for the site.
A strategic site decarbonization plan should be specific in defining opportunities and future performance expectations while leaving flexibility to respond to changes in the operating environment, utility pricing, and legislative frameworks. Plus, we need to recognize that our knowledge of energy-use patterns may improve with increased metering and analytics. A site-specific decarbonization plan should also define the energy engineering specification for new builds at the site, including any technologies to build on a pilot scale for capability building.
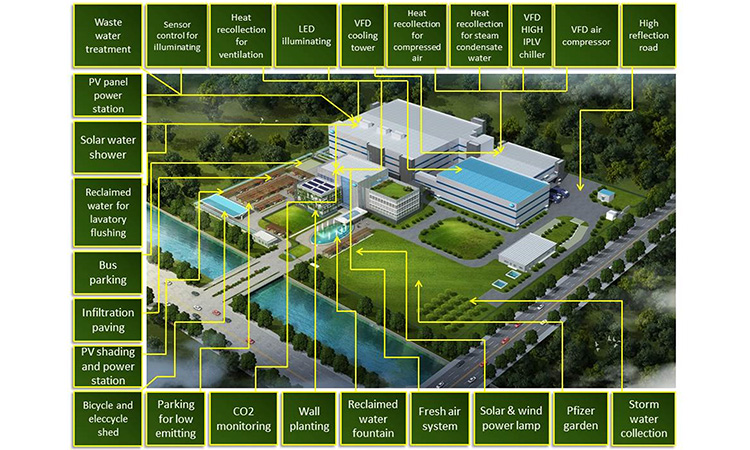
In Europe, ecodesign regulations help us move toward the zero-carbon end point, with HVAC heat recovery now compulsory and fan, chiller, and boiler efficiency requirements driving rapid incremental improvements in new pharma building efficiencies. Figure 1 shows an example of solar power used in HVAC operations for a German site.
Figure 1: Solar collectors delivering hot air to a pharma HVAC dehumidification wheel in Germany. Photo courtesy of Axel Kleuch, Pfizer.