Pharma 4.0™: Hype or Reality?

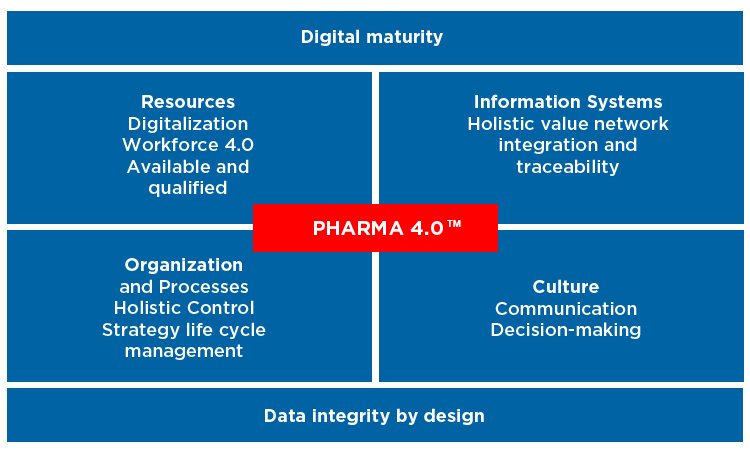
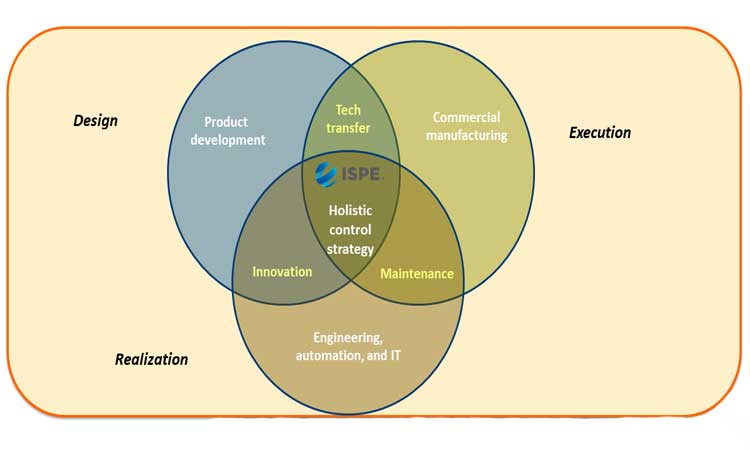
The smart factory, the factory of the future, the Industrial Internet of Things (IIoT), and Industry 4.0. These are buzzwords that populate a new manufacturing world triggered by digitalization. “Pharma 4.0™” is a holistic operating model for pharmaceutical factories and supply chains of the future based on Industry 4.0 capabilities, digital maturity, and data integrity by design1 (Figure 1). Created by ISPE’s Pharma 4.0™ Special Interest Group (SIG), it is fueled by trends such as big data, interconnectivity, collaborative robotics, artificial intelligence, and distributed cloud-based architectures to develop next-generation therapies that may enable lab-to-patient or even patient-to-patient value chains. Pharma 4.0™ is the digitalized operations model of a pharmaceutical organization.
What are the roots of this evolution? What are its philosophies, and how will they influence the future of pharmaceuticals manufacturing?
ROOTS OF THE EVOLUTION
In 2005 Dr. Janet Woodcock verbalized the US Food and Drug Administration (FDA) vision on pharmaceutical risk-based cGMPs for the 21st century as: “A maximally efficient, agile, flexible pharmaceutical manufacturing sector that reliably produces high-quality drugs without extensive regulatory oversight.”2
Additionally, the International Council on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), which supports this vision, pointed out the need for a life cycle approach in ICH Q12.1
PROBLEM STATEMENT
The current single-submission-based control strategy (SSCS) plays a key role in ensuring that critical quality attributes (CQAs) are met and the quality target product profile is realized. This does not, however, enable production-specific changes related to GMP, facilities, utilities, or equipment to mitigate process variability. The effect of unknown process parameters, material attributes, and impurities are often not addressed in the SSCS. Furthermore, it is difficult to foresee these variations over the complete product life cycle during the development phase.
When process and product understanding require a change, this must be communicated to regulatory authorities. It’s also important to refine the control strategy that comes out of development and to enhance it into one that can be executed in manufacturing. Data integrity is still an issue along the pharmaceutical value chain, since repeatable, robust, and right-first-time-based pharmaceutical business processes are not yet fully implemented.
- 2Woodcock, J. “The Concept of Pharmaceutical Quality.” American Pharmaceutical Review 47, no. 6 (2004): 1–3.
- 1International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. “Pharmaceutical Quality System: Q10.” 4 June 2008. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q10/Step4/Q10_Guideline.pdf
Pharma 4.0™ = DIGITALIZATION + ICH Q10
The quality management process in Pharma 4.0™ is based on ICH Q10: “Pharmaceutical Quality Systems” (PQS).1 In 2017, the Pharma 4.0™ SIG published an award-winning article in Pharmaceutical Engineering showing how ICH Q10 can be enriched with “elements and enablers” to benefit from new technologies.3
The four “elements” of the operating model and the “enablers” data integrity by design and digital maturity are shown in Figure 2. This model combines the submission-based and manufacturing control strategies to create a PQS and control strategy that covers the complete product life cycle.
ICH Q10 elements and enablers are shown in grey. New elements made possible by digitalization are shown in blue. When combined with the new enablers digital maturity and data integrity by design, they form a holistic control strategy for the complete product life cycle. This requires information exchange into the decision hierarchy, along the value chain, and across the value network.
ICH-DEFINED ENABLERS
Knowledge management and quality risk management
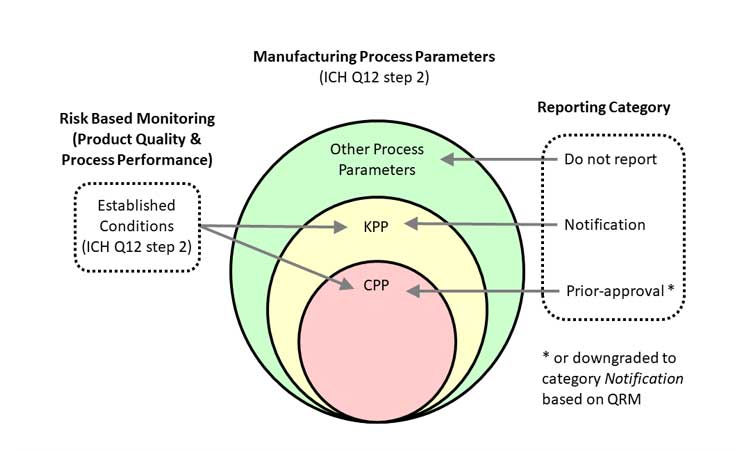
ICH Q10 defines CQAs, critical process parameters (CPPs), and critical material attributes (CMAs) as key elements of product and design. ICH Q12 adds key process parameters (KPPs); these are elements of the manufacturing process that may not be linked directly to CQAs but should be monitored in the move toward a Six Sigma–capable process.
KPPs, CPPs, CQAs, and CMAs are identified by ICH Q12 as established conditions (ECs); these are monitored by product quality and process performance systems to detect out-of-trend or out-of-spec results. ICH Q12 also clarifies the communication necessary between regulatory authorities and firms for changes depending on the type of EC (Figure 3).
Holistic process and platform understanding requires cross-organizational interdisciplinary knowledge management of all suborganizations (internal and external) and integration of all GxP-related information technology (IT) systems. This enables data integrity of all relevant (big) data as well as enhanced analytical approaches that will become the bases for decision-making. Process analytical technology (PAT),7 essential in highly automated environments, further enables advanced technologies like continuous manufacturing.
NEW Pharma 4.0™ ENABLERS
Digital maturity
The four quadrants of an operating model have been common to all stages of industry, but the ways in which they were implemented differed. An organization’s digital maturity defines its capability to operate within the parameters of Industry or Pharma 4.0™. The Pharma 4.0™ SIG has designed a pharma-specific model that allows organizations to assess where they are, which holistic control strategy capabilities are possible, and what the road to future capabilities looks like. Digital maturity is the first enabler in the change to a data-driven, agile organization.
Table A shows that computerization and connectivity are prerequisites for Pharma 3.0. To move toward Pharma 4.0™, an organization needs data visibility, data transparency, predictive capacity, and adaptability. New technologies like paperless execution systems, virtual reality (VR), augmented reality, collaborative robotics, 3D printing, blockchain, and other technologies can empower resources, but will only render value if all four quadrants, which are the Pharma 4.0 operating model elements, are equally mature.
Data Integrity by Design
Data integrity was essential to patient safety even in paper-based eras, so it has always been a focus of regulatory agencies. In Pharma 4.0™, data will travel in all directions of the value network; governing its integrity will pose new challenges. The data pedigree must be transparent, with data flow charts linked to process flow charts.
In the Pharma 4.0™ environment, the performance of business processes along the product life cycle depends on structural capabilities. If an organization is trapped in silos, for example, the chance is high that sociotechnical information systems are designed for and governed by a culture in which each element defends its own “island.” If a holistic control strategy (a “red thread” in popular terminology) is to perform throughout the product life cycle, the structural capabilities that connect to that red thread should be considered during the design, implementation, and operation of the control strategy.
In many organizations business processes are not well defined or documented. But if you want to bring IT into an organization, you must have defined processes and data flows. This starts with the implementation of the enterprise resource planning (ERP) system. Each system that controls the manufacturing process must also be based on a well-structured, documented, and validated software system.
Data integrity is much more than ensuring a good audit trail. It is about data quality, the right content, the data life cycle, and upholding ALCOA+ principles. Excipients, for example, should have just one name and one reference number across the company’s global network to avoid mix-ups and misunderstandings. Data integrity requires well-defined, robust, and repeatable (but flexible) processes, risk-management principles, and critical thinking. It includes thorough data science approaches and architectures. When establishing a quality risk map using ICH Q9, one of the most important steps is risk identification, which requires extensive experience, a balanced view on risk, and foresight on what can go wrong. For this reason, prior knowledge should be available in a structured form.
- 3Herwig, Christoph, et al. “A Holistic Approach to Production Control: From Industry 4.0 to Pharma 4.0.” Pharmaceutical Engineering 37, no. 3 (May-June 2017): 44–49.
- 7US Food and Drug Administration. Guidance for Industry. “PAT: A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance.” September 2004. https://www.fda.gov/downloads/drugs/guidances/ucm070305.pdf

ICH Q10 elements and enablers are shown in grey. New elements made possible by digitalization are shown in blue. When combined with the new enablers digital maturity and data integrity by design, they form a holistic control strategy for the complete product life cycle. This requires information exchange into the decision hierarchy, along the value chain, and across the value network. strategy for the complete product life cycle. This requires information exchange into the decision hierarchy, along the value chain, and across the value network.
NEW Pharma 4.0™ ELEMENTS
Resources
“Resources” refer to tangible, physical resources. These include a company’s workforce (human resources), machinery and equipment, tools, materials, and the final product.4
Physical assets in Pharma 4.0™ will be fast and adaptive, able to produce diverse products with the efficiency of mass production. Smart “plug and produce” equipment will adapt to multiple configurations. PAT will monitor KPPs and communicate through a digital infrastructure with different partners in the value network. New process validation methods will empower continuous improvement.
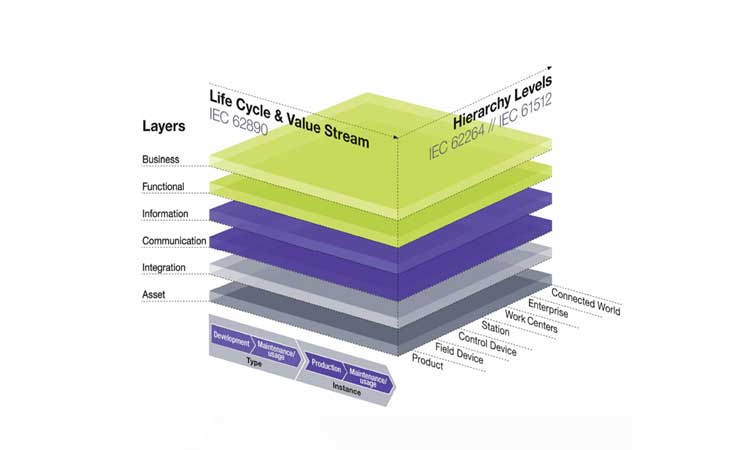
Each product-quality and process-performance monitoring system can be explained with the RAMI 4.0 cube (Figure 4). The integration layer acts as the task-based interface between the digital and the physical worlds (i.e., the human-machine interface). The communication and information layers enable the traceability and visibility of information, which can be pushed to the manufacturer for predictive maintenance through VR and artificial intelligence. Each layer communicates with the whole value chain network, across one site or throughout the entire company. The mature design, implementation, and operation of all axes will reduce latency, enhance the quality and the availability of products, and raise the business benefit.
To excel in this adaptive, information-rich environment, a new breed of human-machine interfaces will ensure flawless data acquisition and information reporting.
- 4Schuh, G., et al., eds. “Industrie 4.0 Maturity Index: Managing the Digital Transformation of Companies.” Acatech Study. Munich: Herbert Utz Verlag, 2017. http://www.acatech.de/fi eadmin/user_upload/Baumstruktur_nach_Website/Acatech/root/de/Publikationen/Projektberichte/acatech_STUDIE_Maturity_Index_eng_WEB.pdf
| Industry | 1.0 | 2.0 | 3.0 | 4.0 |
|---|---|---|---|---|
| Resources | Mechanical | Electrical | Digitalization | Visibility |
| Information systems | Unit operation | Production process | Computerization | Transparency |
| Organization and processes |
Craft shop | Taylorism* | Connectivity | Predictive |
| Culture | Internal focus, adaptive behavior |
Internal focus, stabilizing behavior |
External focus, stabilizing behavior |
External focus, adaptive behavior |
|
* A 19th-century management system that broke down steps in a manufacturing process into repetitive tasks. |
||||
Information systems
Information systems are socio-technical systems in which information is provided based on economic criteria by both people and information and communication technology. They prepare, process, store, and transfer data and information.4
This is the basis for the integration of all supporting computerized systems in Pharma 4.0™, vertically and horizontally across systems, the product life cycle, and the value chain network. This includes data interfaces, process automation to support continuous process verification (CPV) by applying technologies like PAT, and predictive process controls to establish real-time release testing. Recognizing this need, some big pharma companies have established a “data lake” that also serves as “one source” for system integration as well as fast real-time and ad hoc reporting.
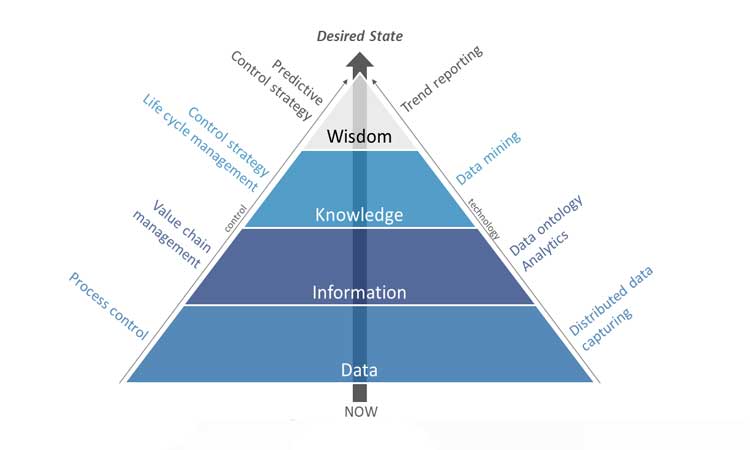
Areas for system integration include preventive maintenance, environmental monitoring, energy management, automation, CPV, mass serialization, real-time release, batch release, and track and trace.3 ERP systems and equipment must also be integrated. Integration concepts must adhere to global technical standards such as GAMP® and ISO. Product development must be oriented toward “manufacturability” in automated processes. Most importantly, good decision-making needs a thorough understanding of data and information, a broad knowledge base, and solid experience (Figure 5).
Organization and processes
Organisational structure refers to both a company’s internal organization (structure and operational processes) and its position within the value network. In contrast to area of “culture,” the “organisational structure” establishes mandatory rules that organize collaboration both within the company and externally.4
In the pharmaceutical industry, which is driven by meeting and complying with regulatory expectations, a holistic control strategy is the key element for life cycle management, followed by a risk-based approach based on well-defined business and pharmaceutical processes.
Process validation guidelines from the ICH1 and FDA5 ,6 recommend flexible production processes, including continued and ongoing process verification, which enables close monitoring of CQAs and CPPs to ensure high product quality.
In Pharma 4.0™, however, the concept of quality assurance must be adapted to cross-functional business processes. In addition, the tasks and responsibilities of systems, cross-functional process owners, and content owners must be redefined.
There is a lot of work ahead of us, and it must be based on a step-by-step approach consistent with other structural capabilities such as culture. If, for example, people need paper documents such as standard operating procedures or working instructions to master the complexity of integration, it becomes obvious that the approach took more than one step, which puts the holistic control strategy at risk.
The long decision chains typical of pharmaceutical organizations should be mitigated through fast, specialized communities, which are created and disbanded depending on the needs of the value chain network. As the network moves toward the Internet of Things or Industry 4.0, pharmaceutical companies should establish cross-functional communities to design the step-by-step approach and ensure the integrity and performance of the holistic control strategy.
Figure 2 shows that Pharma 4.0™ enhances the ICH Q10 PQS with structural organization and processes, creating a new quality by design element in the product life cycle.
Culture
“Culture” covers the value system within the company and thus describes the soft factors of collaboration. Nevertheless, both [organization and culture] structural areas are mutually dependent and must be coherent with each other.4
Implementing Pharma 4.0™ and the holistic control strategy uses the holistic approach to design and execute the business processes and to bring automation and paperless execution to the shop floor. This requires a culture of collaboration for all business units (Figure 6) responsible for the production process, technology, and quality. Some regulatory authorities have started to request control strategy digitalization. This request absolutely makes sense considering that the holistic control strategy implementation uses more and more IIoT and Industry 4.0 solutions.
Organizational culture should be geared toward understanding the importance of each element in the control strategy:
- Audit trails along critical information flows are designed to detect design-space changes—not to control human beings.
- If one department does not produce risk-based evidence for some human activities, the integrity of the holistic control strategy is jeopardized.
- Stakeholders should not wait to request improvements in their organizational culture, the use of socio-technical information systems, and greater automation of resources.
It is management’s responsibility, according to ICH Q10, to ensure compliance with the holistic control strategy.
- 4 a b
- 5US Food and Drug Administration. Guidance for Industry. “Process Validation: General Principles and Practices.” January 2011. http://www.fda.gov/downloads/Drugs/Guidances/UCM070336.pdf
- 6US Food and Drug Administration. Guidance for Industry. “Q8, Q9, and Q10: Questions and Answers (R4).” ICH Revision 1. November 2011. https://www.fda.gov/downloads/Drugs/Guidances/UCM210822.pdf
SUMMARY
There is a huge potential for improving safety, quality, transparency, agility, flexibility, and productivity by implementing the Pharma 4.0™ holistic control strategy across the pharmaceutical value network. The regulatory framework defined in ICH and FDA guidance is a prerequisite to ensure patient safety and stakeholder benefit.
Once this has been established, all that is needed is the entrepreneurial courage to start and the guidance to change with a controlled road map.
Acknowledgments
The Pharma 4.0™ SIG of the ISPE Germany, Austria, and Switzerland (DACH) Affiliate would like to thank the following Pharma 4.0™ leaders and subgroup leaders: Christian Wölbeling, Werum (Chair); Hans Heesakkers, Circuition (Co-Chair); Jennifer Baughman, MilliporeSigma; Lorenz Binggeli, B. Braun Medical; Wolfgang Dedden, Bayer; Uli Kuchenbrod, Vetter Pharma-Fertigung; Volker Roeder, Arcondis; Klaus Sauermann, Werum; and Thomas Zimmer, ISPE.
The Pharma 4.0™ SIG and its subgroups aim to provide industry-wide implementation methodologies, approaches, and case studies on how to evolve life science organizations to Pharma 4.0™. The group is currently focusing on the first step required to implement digital maturity into a pharma organization: Make it think and act in well-defined, best practice business process, and follow a data integrity by design approach.
They will post progress reports on a regular basis.


