Women in Pharma® is a place where women and men—especially those new to the industry—can access a network of mentors, role models, and educational resources to support their professional success. The widespread global interest...

Downloads
Women in Pharma®: Empowering Women as Industry Leaders
Cover: Women in Pharma® is a place where women and men—especially those new to the industry—can access a network of mentors, role models, and educational resources to support their professional success. The widespread global interest and participation in this initiative and its events have shown that women in the pharmaceutical industry are hungry for the connection, mentoring, and education that Women in Pharma offers.
Creating Effective Standard Operating Procedures
Feature: A standard operating procedure outlines agreed-upon instructions for personnel training and instructions for maintaining systems, machines, documents, and records in a qualified state to produce safe products. This article explores the role of SOPs, as well as their structure and components.
Industry Perspective: Model-Informed Drug Development Addresses COVID-19 Challenges
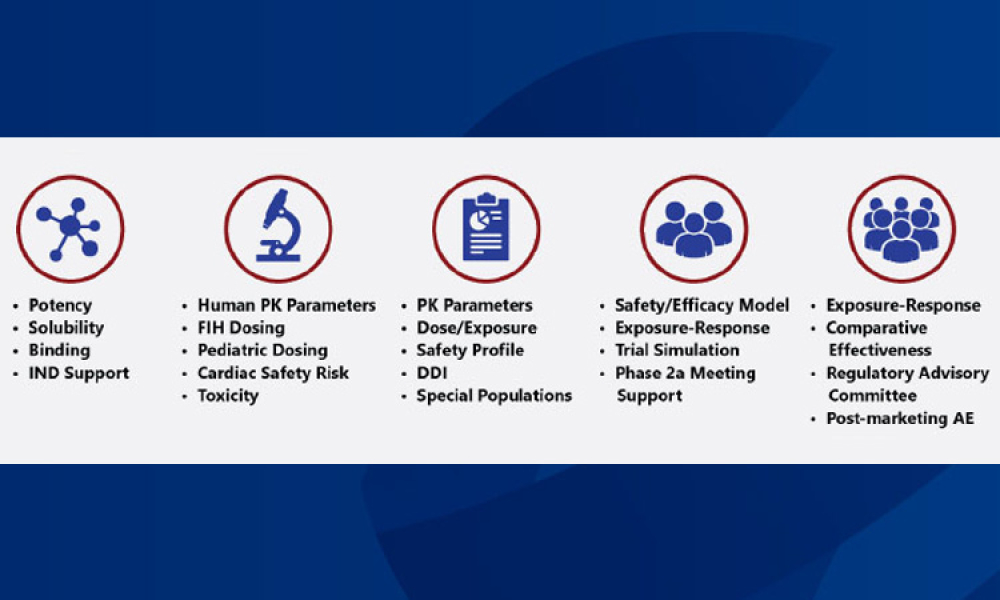
How can drug developers increase the efficiency and effectiveness of drug development? One useful tool is model-informed drug development, which uses computer models to inform the design of clinical trials or to run simulations when human or animal trials are not feasible. By ensuring that appropriate drugs are advanced and the clinical trial design is optimized, MIDD helps drug companies develop therapies for emerging diseases like COVID-19.
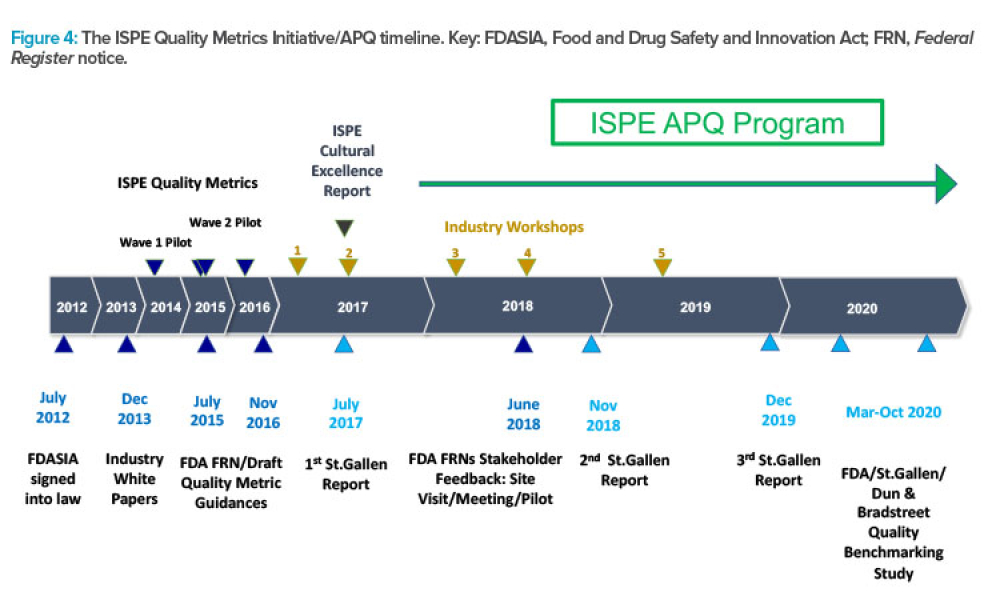
ISPE’s APQ Program and Guides Advance Pharmaceutical Quality
Feature: ISPE has announced the launch of its Advancing Pharmaceutical Quality Program with the publication of the ISPE APQ Guide: Corrective Action and Preventive Action (CAPA) System, a guide dedicated to the topic of CAPA. This article describes how the APQ Program has been built and summarizes the content covered in the Advancing Pharmaceutical Quality Guide series, using the CAPA guide as an example.
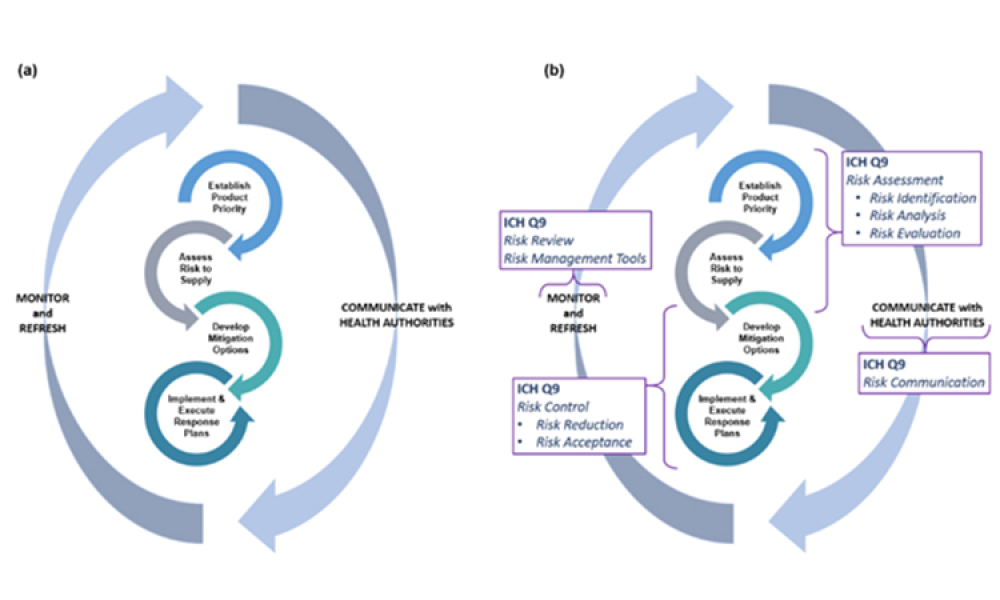
Quality Risk Management to Address Product Impurities
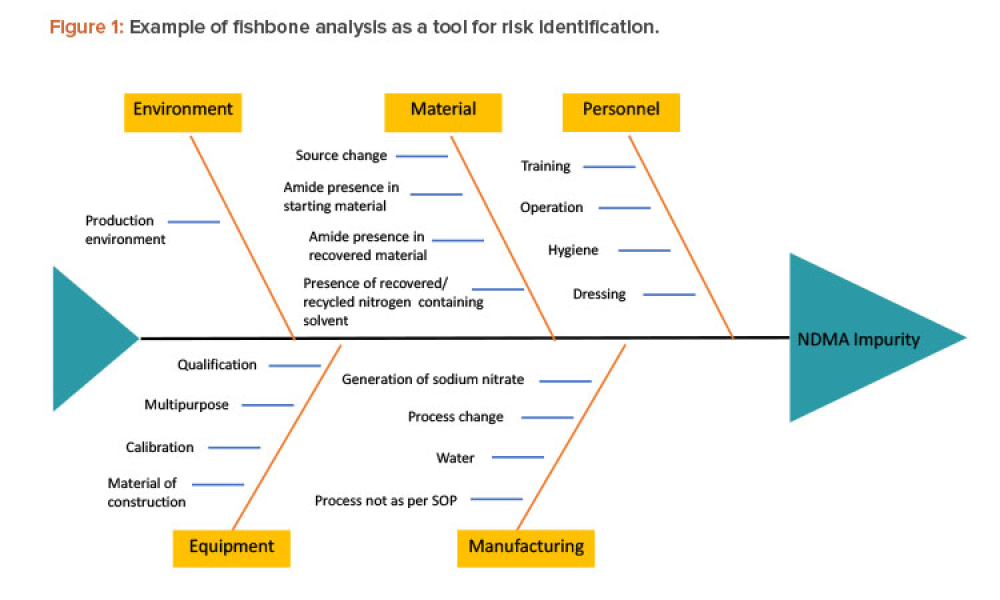
Technical: Recently, recalls of angiotensin receptor antagonists, particularly valsartan, and warning alerts about N-nitrosodimethylamine impurities in drug substances such as ranitidine and metformin have demonstrated the urgent need for manufacturers and regulators to control impurities throughout the product life cycle to ensure patient safety. This article discusses all plausible pathways related to the formation of NDMA impurities in pharmaceutical products and a possible control strategy using quality risk management as a tool.
In This Issue
I was a skeptic. When the Women in Pharma® (WIP) program started in 2016, I had previously attended several other professional women’s programs and usually left disappointed, as these programs did not provide the information I...
I think everyone will join me in celebrating the start of 2021! With all the challenges brought by 2020, last year also demonstrated the resilience and innovative thinking of ISPE members. With collaboration and perseverance in mind, I was delighted to begin my tenure as ISPE International Emerging Leaders Chair at the virtual
My parents always told me when I was growing up that I could be anything I wanted to be. They strongly steered me in the direction of engineering at North Carolina State University, so that I would be able to earn a degree that would ensure my future was my own. When I was eight years old, I was introduced to a pharma company my dad worked for and you could say my love of this industry has...
Data Integrity by Design is the concept that data integrity must be incorporated from the initial planning of a business process through to the implementation, operation, and retirement of computerized systems supporting that business process.
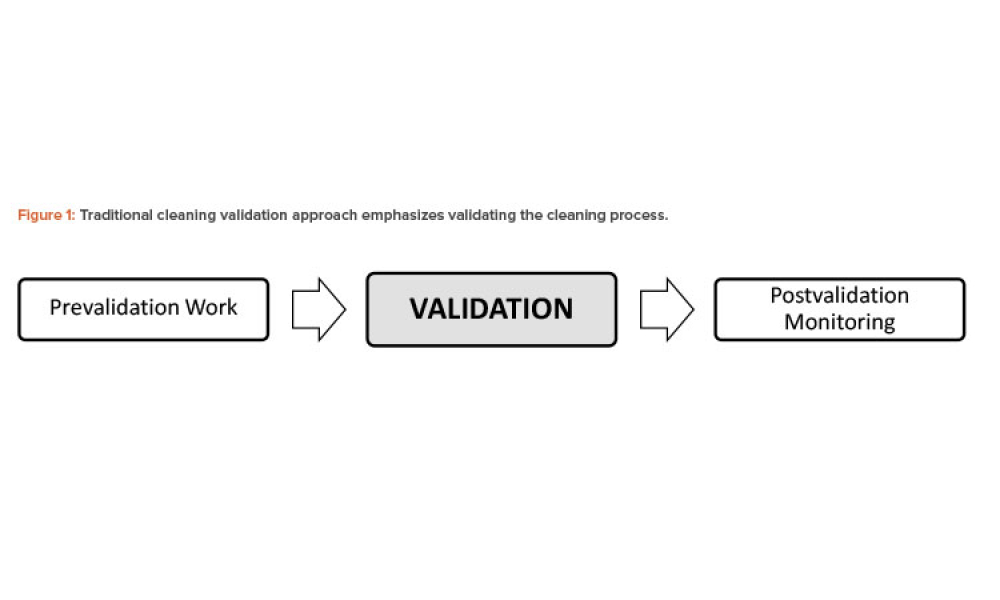
The process life-cycle model, as discussed in the US FDA guidance on process validation, is a significant change in how we view validation.1
Personnel management is the most challenging variable in maintaining current Good Manufacturing Practice (cGMP) across the life cycle of drug manufacture, safety, and supply. A standard operating procedure (SOP) outlines agreed-upon instructions for personnel training and instructions for maintaining systems, machines, documents, and records in a qualified state to produce safe products. This...
Drug developers know that the odds of anyone compound demonstrating safety and efficacy for a disease and its affected populations are low. How can drug developers improve these odds and increase the efficiency and effectiveness of drug development? One useful tool is model-informed drug development (MIDD), which uses computer models to inform the design of clinical trials or to run...
Recently, recalls of angiotensin receptor antagonists, particularly valsartan, and warning alerts about N-nitrosodimethylamine (NDMA) impurities in drug substances such as ranitidine and metformin have demonstrated the urgent need for manufacturers and regulators to control impurities throughout the product life cycle to ensure patient safety.1
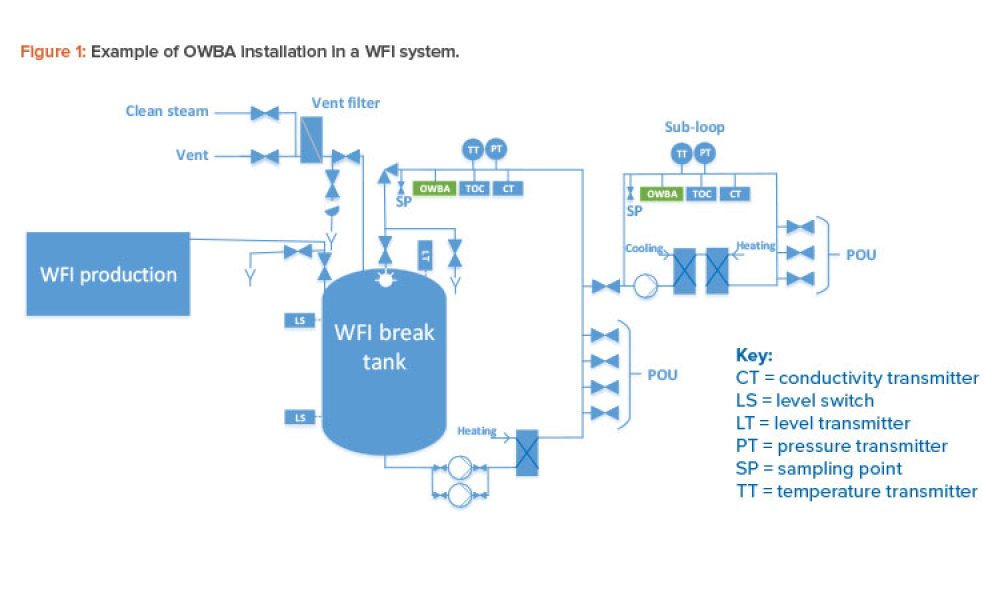
Online water bioburden analyzers (OWBAs) are analytical instruments providing real-time or near real-time measurement of bioburden in purified water systems.1
The 2020–2021 ISPE International Board of Directors began their term at the Member Meeting on 5 November during the
ISPE has announced the launch of its Advancing Pharmaceutical Quality (APQ) Program with the publication of the ISPE APQ Guide: Corrective Action and Preventive Action (CAPA)...
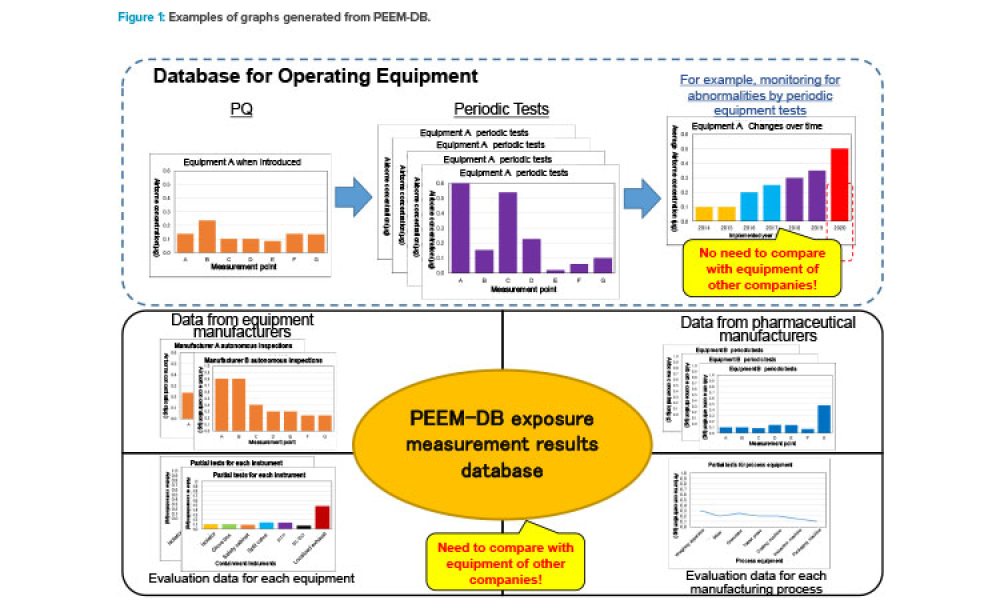
This article describes the Pharmaceutical Equipment Exposure Measurement Database (PEEM-DB), which was launched in July 2019 by the ISPE Japan Affiliate for its members. PEEM-DB is offered as a tool for rationally advancing optimal containment equipment settings by collecting exposure measurement results for...