Effect of Low-Energy E-Beam Irradiation on Presterilized COC Packaging

Does electron-beam surface decontamination radiation damage COC syringes? Experimental investigations confirm that no measurable dose is delivered if irradiation parameters are selected correctly. Even a dose of a few kGy (equivalent to a few 0.1 Mrad) would not cause significant change.
Aseptic filling of sterile drugs is a critical process in biopharmaceutical manufacturing. Ready-to-use pre-sterilized syringes must be transferred into the isolator for filling. Electron-beam (e-beam tunnel) radiation that decontaminates the outer surfaces of the tubs containing pre-sterilized syringes (and other containers) is generally seen as a best practice solution for high-speed filling lines. Figure 1 shows a typical combination of an e-beam tunnel and a filling line.
The typical e-beam tunnel contains three electron accelerators, called e-beam emitters, arranged in a triangular configuration for optimal decontamination of all surfaces (Figure 2). The tubs move on a conveyor belt through the electron cloud generated by the e-beam emitters. Electrons in the 100-to-150-kiloelectron-volt (keV) energy range have very limited penetration power. Using a minimum surface dose of 15 kilogray (kGy)* on all external surfaces leads to a greater than 6-log reduction of colony-forming units.1 Before the decontamination process can start, however, the entire e-beam tunnel (and possibly the isolator as well) must be decontaminated with hydrogen peroxide gas (H2O2).
* Gy (100 rad) is defined as the absorption of one joule of radiation energy per kilogram of matter (1 J/kg).
- 1Miller, Arne, et al. “Guide on the Use of Low Energy Electron Beams for Microbiological Decontamination of Surfaces” Presented at the Panel on Gamma and Electron Irradiation, London, October 2013. http://orbit.dtu.dk/fi les/60269574/Guide_on_the_use.pdf
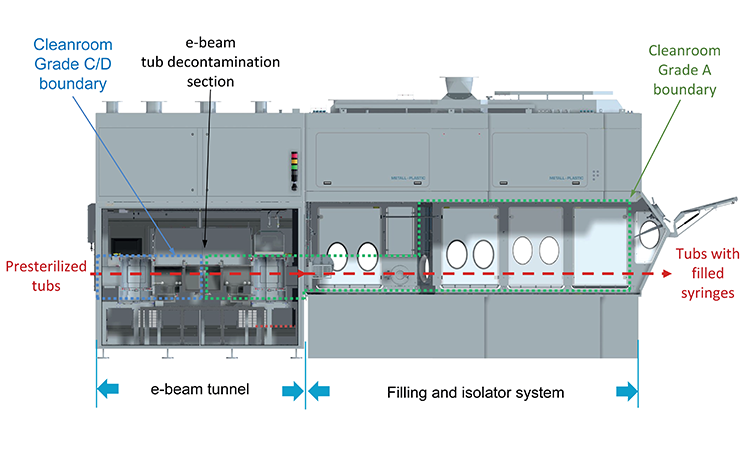
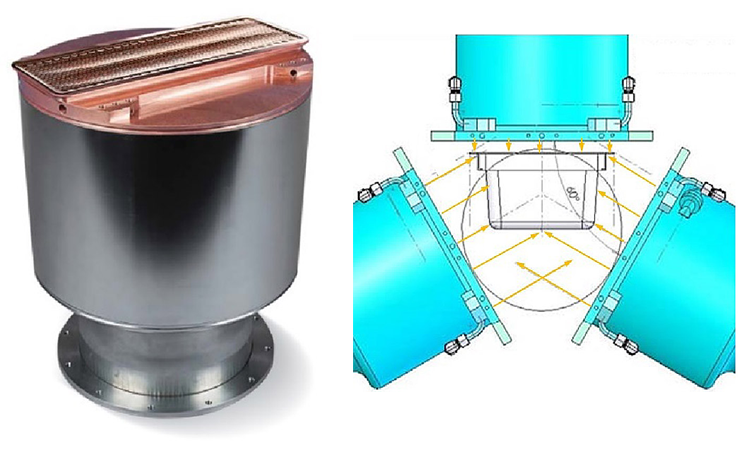
E-beam tunnel irradition ensures that the aseptic zone in the filling area remains uncompromised. Its main benefits over alternative techniques such as rapid transfer port, high-intensity ultraviolet light surface sanitization, and double debagging are high microbial kill efficacy, throughput of up to six tubs per minute, well-defined dose and validation requirements, and few control parameters (voltage and current of e-beam emitters and speed of the tubs moving through the e-beam zone).2
E-beam tunnels are well established for aseptic filling, with more than 30 units in operation worldwide. In the past, they were used to decontaminate tubs containing glass syringes. Because it is possible that some radiation might penetrate the tub and damage the syringe material, objectives included avoiding glass discoloration and preventing ozone accumulation inside the syringes.3
Recently, however, pharmaceutical companies have begun to use polymer syringes made of cyclic olefin copolymer (COC). But the behavior of this material when irradiated is not as well understood as that of glass. It was important, therefore, to investigate the decontamination of tubs containing COC syringes.
EVALUATION
As already mentioned, the purpose of the e-beam tunnel is to decontaminate the outer surfaces of the tub. Because radiation can penetrate the tub and the syringes, however, we must determine the dose (if any) delivered to the syringes and assess the damage (if any) to the COC material. For this purpose, it is important to understand the physical design of the tub.
As depicted in Figure 3, the syringes are contained in a nest, and covered with a Tyvek liner. The nest sits in a polystyrene tub that has a Tyvek lid glued to its edges.
In addition to e-beam radiation, an e-beam emitter produces an extremely small dose of x-ray radiation as the electrons are stopped in the titanium foil or the copper support of the electron window. Our measurements showed these values to be below 0.2 Grays per second (Gy/s), equivalent to 20 rads per second (rad/s) inside the tub. Given an exposure time in the e-beam radiation zone of approximately 10 seconds, the deposited dose amounts to only a few Gy (100 rad) at most, or approximately 0.1% of the dose needed to decontaminate the outer surfaces. Therefore, no significant effect can be expected, but the x-ray dose must be added to the electron dose when evaluating the whole effect of the radiation.
- 2Vogt, O. “Case Study: Utilizing Electron beam Surface Decontamination to Transfer Sterile Syringe Barrels into an Isolated Aseptic Syringe Filling Line.” Pharmaceutical Engineering 30, no. 1, (January-February 2010).
- 3Morisseau, Didier, and Fiona Malcolm: “SterStar System: Continuous Sterile Transfer by E-Beam.” Radiation Physics and Chemistry 71 (2004): 553–556.

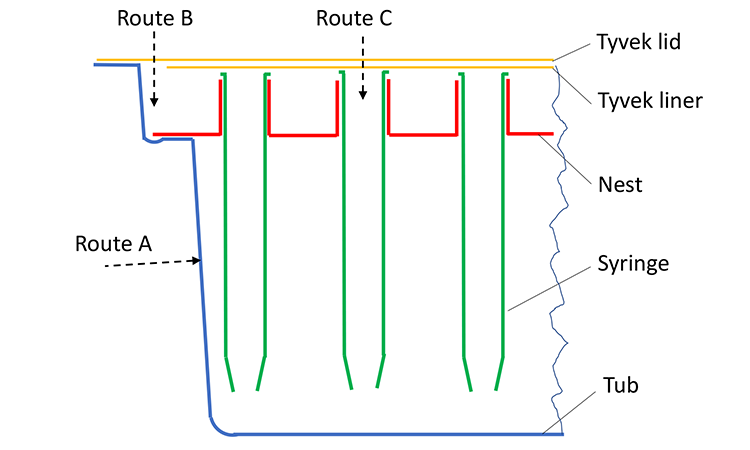
We must also determine the penetration capabilities of low-energy electrons and examine the different routes by which they might enter the tub. Given the physical design of the tub, one can find three entry routes (Figure 4).
We measured the depth dose using the RisøScan dosimetry tool4 and the Dμ concept of low-energy electron dosimetry.5 As Figure 5 shows, penetration in a material with a density of 1 gram per cubic centimeter at a voltage of 150 kilovolts (kV) is 0.25 millimeters (mm). At 120 kV it is 0.16 mm, when the distance between emitter and material is 20 mm.
Route A is through the side walls or the bottom of the tub. Given a wall thickness of 0.8 to 1.5 mm—which is greater than the 0.25 mm penetration even at a maximum voltage of 150 kV—no electrons will penetrate the walls of the tub, and no radiation will be deposited on the syringes.
Route B is through the lid and into the gap between tub liner and wall (Figure 6); following this route, the electrons will hit the nest, and thus will not deposit any dose on the syringes.
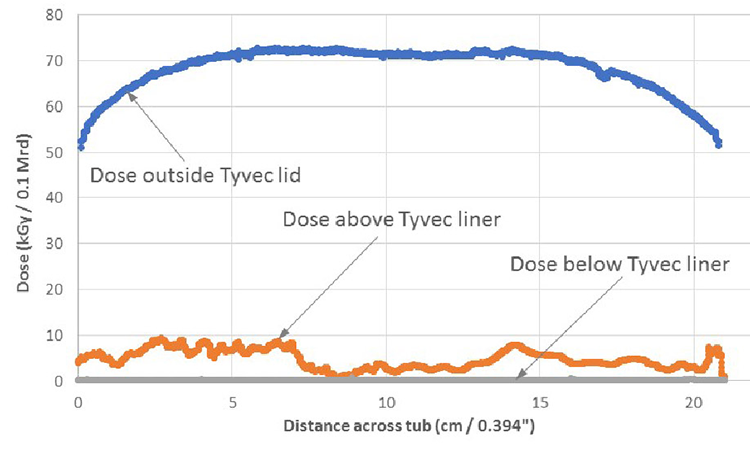
Route C is through the outer Tyvek lid and the inner Tyvek liner. The medical and pharmaceutical packaging foils typically used (1073B, 1059B, and 2FS) have basis weights of 59.5 grams per square meter (g/m2) to 74.6g/m2.6 Using one Tyvek foil as lid and a double Tyvek foil as liner, the total basis weight is between 178.5 g/m2 and 223.8 g/m2. With a voltage of 120 kV (typical voltage ranges between 100 and 115 kV) on the e-beam emitter facing the top of the tub, the penetration in terms of basis weight in the Tyvek foil will be 160 g/m2 (Figure 7). For this configuration, no electrons will enter the tub.
We must also consider the nonhomogeneous thickness of the Tyvek foil. To assess whether this might transmit some radiation to the syringes, we conducted an investigation using a tub configuration and voltage used by pharmaceutical companies.
We selected a tub with a lid of basis weight 87.3 g/m2 and a single liner of 76.1 g/m2, and irradiated it with a voltage of 115 kV, measuring the doses above the lid as well as above and below the liner. Figure 7 shows that below the liner, which touches the top of the syringes, there is no measurable dose (i.e., it is below the 0.2 kGy measurement sensitivity threshold of the dosimetry tool). In other words, if the parameters (voltage and foil thickness) are selected correctly, then the dose on the syringes will be close to zero.
- 4Helt-Hansen, Jakob, and Arne Miller: “RisøScan—A New Dosimetry Software.” Radiation Physics and Chemistry 71, no. 1–2 (September–October 2004): 361–364.
- 5Helt-Hansen, Jakob, et al. “Dμ—A New Concept in Industrial Low-Energy Electron Dosimetry.” Radiation Physics and Chemistry 79, no. 1 (January 2010): 66–74.
- 6E. I. du Pont de Nemours and Company. “Technical Reference Guide for Medical and Pharmaceutical Packaging.” http://www.dupont.com/content/dam/dupont/products-and-services/packaging-materials-and-solutions/medical-and-pharmaceutical-packaging-materials/documents/DPT_MPP_Technical_Reference_Guide.pdf
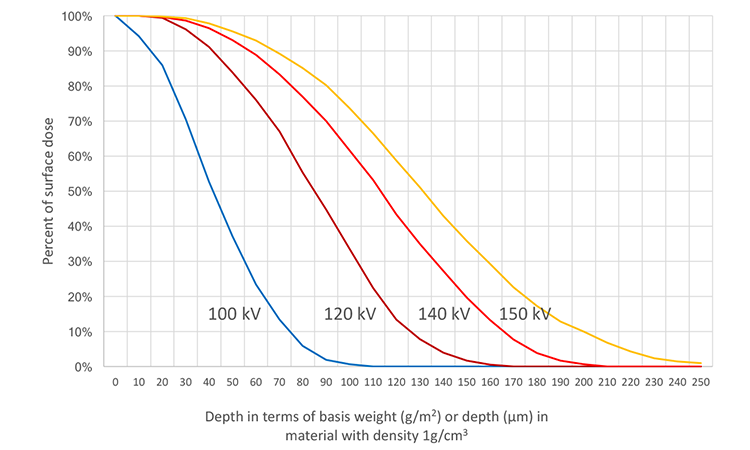

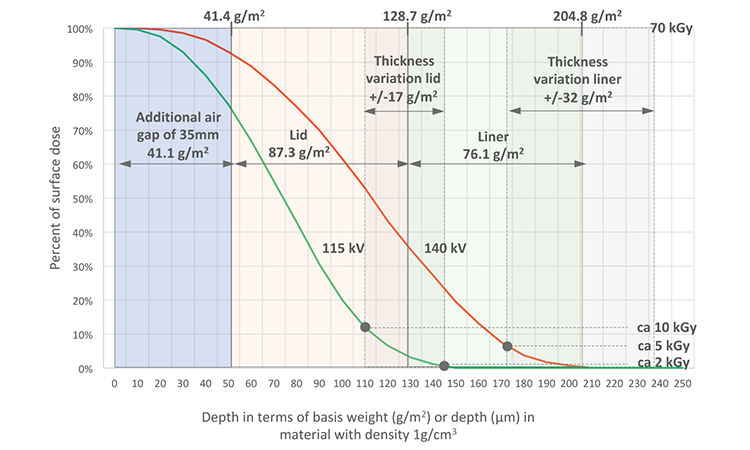
But what if unsuitable parameters are chosen, such as a very thin liner or a voltage that significantly exceeds 120 kV? To estimate the radiation dose that might be delivered under these conditions, we assumed the same foil thicknesses as in the initial investigation, but increased the voltage to 140 kV. We then used a simple graphical method based on the depth dose curves, which is commonly used with homogeneously thick material by users of e-beam equipment (Figure 8).
We overlaid the depth dose curves with rectangles that represent the different objects in front of the electron window. In the initial investigation they include the additional air gap of 35 mm (total air gap 55 mm, air gap of 20 mm already considered in the depth dose curves) converted to a basis weight of 41.1 g/m2, the lid with a basis weight of 87.3 g/m2, and the liner with a basis weight of 76.1 g/m2.
E-beam tunnels are well established for aseptic filling, with more than 30 units in operation worldwide."
The initial investigation had shown a below-lid dose variation of approximately 2–10 kGy, which implies a thickness variation of the lid of approximately ±17 g/m2. This allows us to assume a correspondingly higher thickness variation for the combination of lid and liner of ±32 g/m2. Figure 8 shows that the area of lowest thickness of the combination of lid and liner received a dose of approximately 5 kGy (0.5 Mrad). Based on this maximum dose below the liner (and therefore on the syringes) we can evaluate the changes to the characteristics of the syringes, drawing on research by other groups.
COC material damage might occur in two ways: 1) directly, from low-energy electrons or 2) indirectly, from irradiation of the air in the tub, which leads to the formation of ozone (O3), nitric acid (HNO3), and nitrogen oxides (NOx), all of which oxidizing gaseous agents.1
The direct effect of electron-beam irradiation leads to chain scission, cross-linking, oxidation, and grafting. These in turn may change the mechanical or surface properties of the polymer, leach low-molar mass molecules from the polymer into the drug solution, allow the polymer to absorb the drug, or affect the compatibility of the packaging and its content.7 ,8
"E-beam tunnel irradiation ensures that the aseptic zone in the filling area remains uncompromised.”
But does a moderate dose of no more than 5 kGy (0.5 Mrad) lead to significant damage on COC packaging? Because research is usually conducted at high doses, typically between 25 and 200 kGy, we must interpolate between the points of no irradiation and irradiation at 25 kGy. Two papers by Barakat report measurements on modifications of the characteristics of COC with respect to polymer degradation, effect on the antioxidant degradation, effect on the generation of low-molecular-weight compounds, chemical modifications, and interaction with drug solutions. All measurements showed only a small difference between the points of no dose and a dose of a few kGy.7 ,8 This means that no significant changes to the COC polymer characteristics would be expected.
- 7 a b Barakat, H., et al. “Effect of Electron Beam Radio Sterilization on Cyclic Olefin Copolymers Used as Pharmaceutical Storage Materials.” Radiation Physics and Chemistry 84 (March 2013): 223–231.
- 8 a b Bakarat, H., et al. “Modification of a Cyclo-Olefin Surface by Radio-Sterilization: Is There Any Effect on the Interaction with Drug Solutions?” International Journal of Pharmaceutics 456 (1 November 2013): 212–222.
Conclusion
We have investigated the effects of x-ray and e-beam radiation on syringes made of COC material located inside a tub. Very low doses of x-ray radiation (a few Gy or 100 rad) are negligible in comparison with estimated e-beam dose of 5 kGy (0.5 Mrad) in case of unsuitable parameters.
Experimental measurements showed that for well-chosen parameters the e-beam radiation delivered to syringes inside the tub will be close to zero. Even in case of unsuitable parameters, a dose of 5 kGy (0.5 Mrad) would still not produce significant changes to the COC polymer characteristics, as it has been shown by other groups.7 ,8
- 7Barakat, H., et al. “Effect of Electron Beam Radio Sterilization on Cyclic Olefin Copolymers Used as Pharmaceutical Storage Materials.” Radiation Physics and Chemistry 84 (March 2013): 223–231.
- 8Bakarat, H., et al. “Modification of a Cyclo-Olefin Surface by Radio-Sterilization: Is There Any Effect on the Interaction with Drug Solutions?” International Journal of Pharmaceutics 456 (1 November 2013): 212–222.


