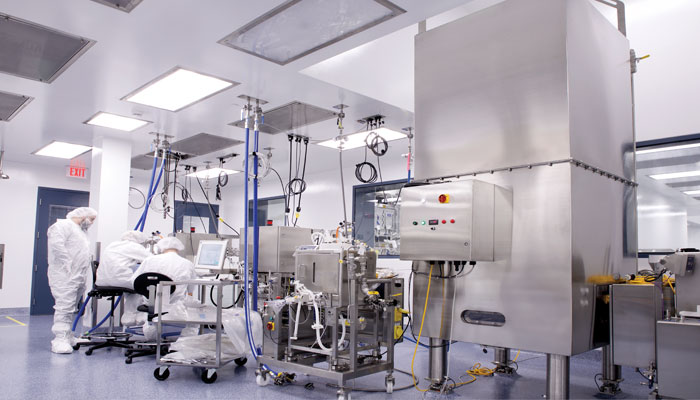
Meet BioMarin Pharmaceuticals, Inc – 2018 FOYA Project Execution Category Winner


BioMarin Pharmaceuticals is the 2018 Facility of the Year ® Project Execution Category Winner for their Project FAITH site in Novato, CA.
Projects selected in this category exemplify the application of novel tools and approaches to delivering projects that improved efficiencies, overcame unusual challenges, promoted effectiveness, and organized stakeholders and project team participants in ways that led to successful outcomes.
For the past two decades, BioMarin has kept its innovation focused on “Finding Tomorrows” for those afflicted by serious and life-threatening rare disorders. Celebrating its 20th anniversary in 2017, BioMarin has grown to a global scale as a biotechnology development leader, with great experience commercializing innovative therapies that focus on the unmet needs of patients – primarily children.
The company’s development pipeline is robust, with multiple clinical and pre-clinical product candidates treating rare genetic diseases. Among the candidates in its pipeline is valoctocogene roxaparvovec, BioMarin’s investigational gene therapy for hemophilia A which has the potential to be the first gene therapy for severe hemophilia.
Gene therapies are designed to remedy a genetic problem by adding a corrected copy of the defective gene. The functional gene is inserted into a vector – containing a DNA sequence coding for a specific protein – that acts as a delivery mechanism, providing the ability to deliver the functional gene to cells. The cells can then use the information to build the functional protein that the body needs, potentially reducing or eliminating the cause of the disease. BioMarin’s investigational program is driven by the potential to impact a patient community seeking to achieve normal steady state.
Announcing the clinical trial results of its gene therapy, BioMarin said the company was ready to advance valoctocogene roxaparvovec development quickly, pointing to the commissioning of its new commercial gene therapy manufacturing facility at its Novato, California campus as one of the fundamental reasons why the company is prepared to start Phase III trials.
In the context of this urgency, BioMarin, along with its A/E and general contracting partners, embarked on “Project FAITH” to develop the capacity BioMarin believed would be needed. However, to execute the company’s strategic and accelerating product plans, the new facility would have to be developed, built and commissioned within a constrained period of time. BioMarin, rejecting early green field-oriented capacity concepts, opted to transform existing warehouse capacity at its Novato site into the world-class, advanced biologics processing and finishing facility required to manufacture doses of valoctocogene roxaparvovec and fill it into vials. The caveat was that the project would have to be completed within a fast-shrinking deadline of just 18 months – which is where FAITH came in!
Knowing that time-to-market is everything, BioMarin needed to expedite the
program. In order for the project to be successful, it was necessary to deliver 18,000 sq. ft. of controlled cell culture and manufacturing space, QC testing capabilities, and the necessary aseptic fill and finish capacity in time to meet product strategy. This meant construction and engineering teams had just 11 months. Less than a year later, this aggressive schedule had been met and BioMarin was manufacturing Phase III clinical batches of both doses of the product as of October 20, 2017.
The execution of Project FAITH represents a spectacular display of pharmaceutical engineering and professionalism. The central reason BioMarin’s Project FAITH embodies the ideals of FOYA is because it demonstrates how, when teamed with some of the industry’s best people, equipped with the right technology, and energized by the right motivation, an impossible project is made possible. The timeframe alone indicates how well the project was executed. From design to construction to schedule and change control, three parties involved – BioMarin; Project Architect and Engineer, CRB; and General Contractor, NOVO Construction – brought an intensive professionalism to Project FAITH that ultimately achieved success. Project FAITH created a common, shared belief among all its participants that with will, wisdom and professionalism, they could achieve one the fastest and most successful implementations of new biopharmaceutical manufacturing capacity ever seen in the industry. The precedents and practices established by this project may influence the delivery of biopharmaceutical innovation and support of the supply chain for years to come.
ISPE congratulates the BioMarin Pharmaceuticals team for their achievements and their FOYA award-winning entry for Project Execution. Learn more about the 2018 FOYA Project Execution Category winner.
Has your company recently designed, built or renovated a state-of-the-art pharmaceutical or biotechnology facility that is best in its class? ISPE is now accepting entries into the 2109 ISPE Facility of the Year Awards (FOYA) Program, and your facility may be eligible to apply. Learn how your company can win.
Facility of the Year Awards Banquet
Join ISPE and prominent industry leaders as we recognize the 2018 Facility of the Year Awards (FOYA) Category Winners for their innovation and creativity in pharmaceutical and biotechnology facility design, construction, and operation, at the FOYA Banquet on the evening of Sunday, 4 November 2018 in Philadelphia, PA during the ISPE Annual Conference.