The following blog post was provided by Peyton Myers, an undergraduate student at Appalachian State University. Myers attended the 2023 ISPE Annual Meeting & Expo in Las Vegas as an ISPE Foundation Professional Development Grant recipient.

Our goal with this redesign was to provide our Members and potential Members with an interactive, fluid means to access all that ISPE has to offer—from regulatory resources, trending pharma news, conferences, trainings, guidance documents, and more.
Immediately you will notice streamlined navigation, fresh, clean design, and a responsive layout. All of this allowing you to access the information you need easily—anytime, anywhere.
Here are the six biggest beneficial user improvements:
1. Clean and Fresh Layout: As ISPE’s global presence continues to thrive, we want our website to reflect that growth. The modern design has a crisp, global look and feel to appeal to all users worldwide.
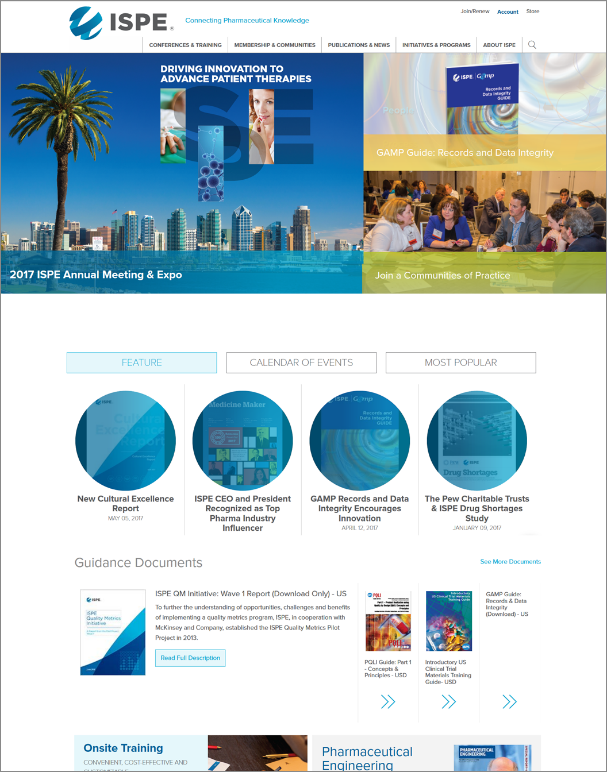
2. Easy Navigation: To go hand-in-hand with the fresh design, the new website was crafted with a clear, direct navigational structure to enable you to easily find the resource and/or content you are looking for. The updated homepage leads with three key facets: Feature, Calendar of Events, and Most Popular.
3. Enhanced Searchability: You will have the entire website at your fingertips with advanced searching capacities, offering more relevant and complete search results.
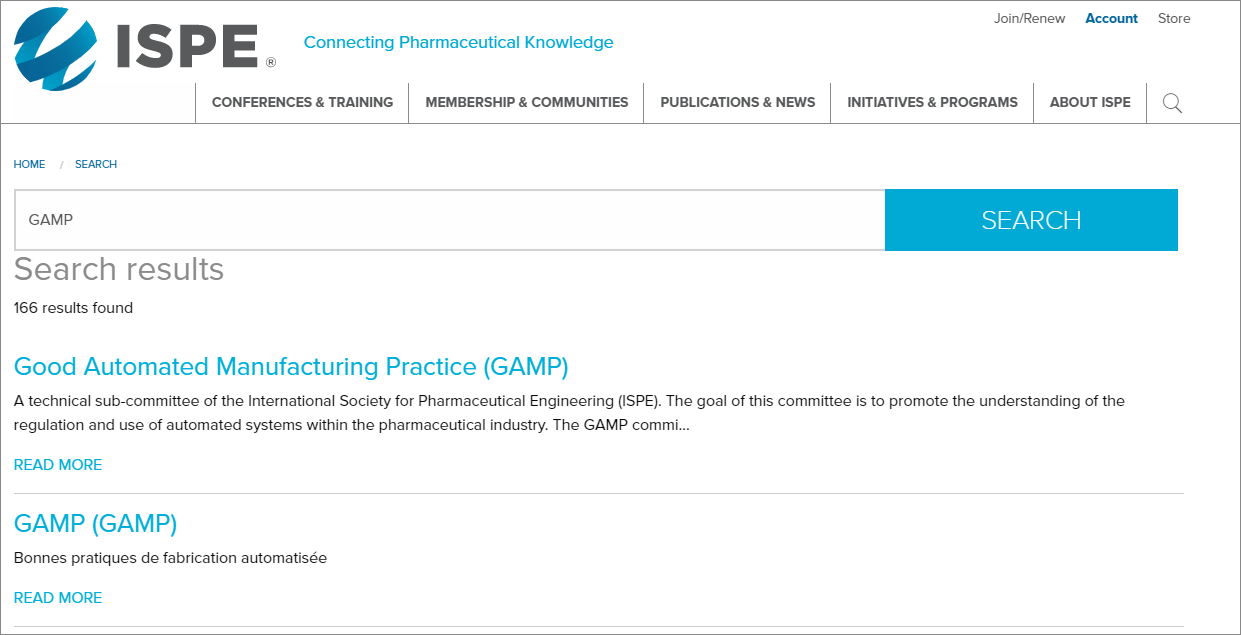
4. Interactivity: A highly interactive design will keep you interested in learning more about what ISPE has to offer you and the pharmaceutical industry. The content under the Most Popular section is user generated, so what is shown is based on what’s trending on the ISPE website. In addition, easily and instantly share content on all sub-pages using the Share bar featuring various email and social media platforms.
5. Responsive: No matter where you are, you will be able to easily navigate the website from any phone, tablet, or other mobile devices.

6. Accessibility: Effortlessly gain access to ISPE’s local and topic-specific resources, including Chapter, Affiliate, and Communities of Practice (CoP) sites.
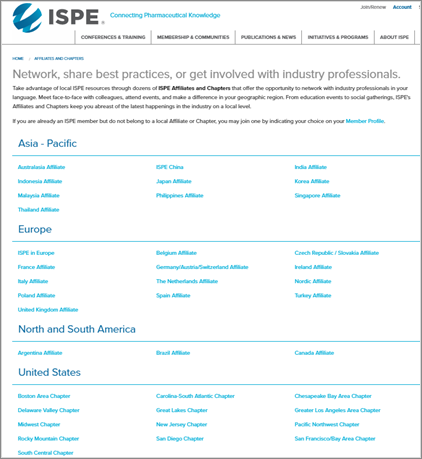
We hope you enjoy exploring ISPE’s new website, learning more about our full spectrum of resources available to support your professional initiatives and those of the pharmaceutical manufacturing industry.
We Want to Hear Your Feedback! Let us know if you have any questions or suggestions about our new website by commenting below.
The following blog post was provided by Peyton Myers, an undergraduate student at Appalachian State University. Myers attended the 2023 ISPE Annual Meeting & Expo in Las Vegas as an ISPE Foundation Professional Development Grant recipient.
The integration of data science in biopharmaceutical manufacturing, emphasizing data quality, tech transfer efficiency, and process optimization, is the heart of this track. Led by industry experts, discussions explore leveraging digital twins, predictive analytics, and continuous improvement initiatives. Additionally, interactive roundtable discussions provide attendees with a dynamic forum...
The pharmaceutical sector stands at a crossroads of immense possibilities. We are witnessing an unprecedented surge in innovation, fueled by a remarkable partnership between industry and regulatory authorities. For example, there are initiatives fostered by the US Food and Drug Administration (US FDA) to promote innovation with programs such as CATT (CBER Advanced Technologies Team) and ETP...