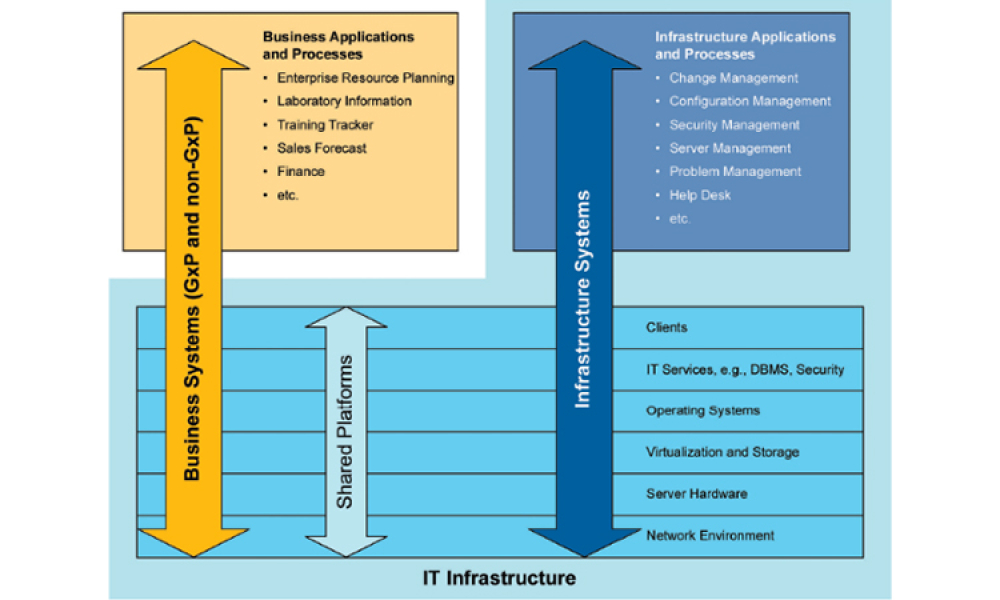
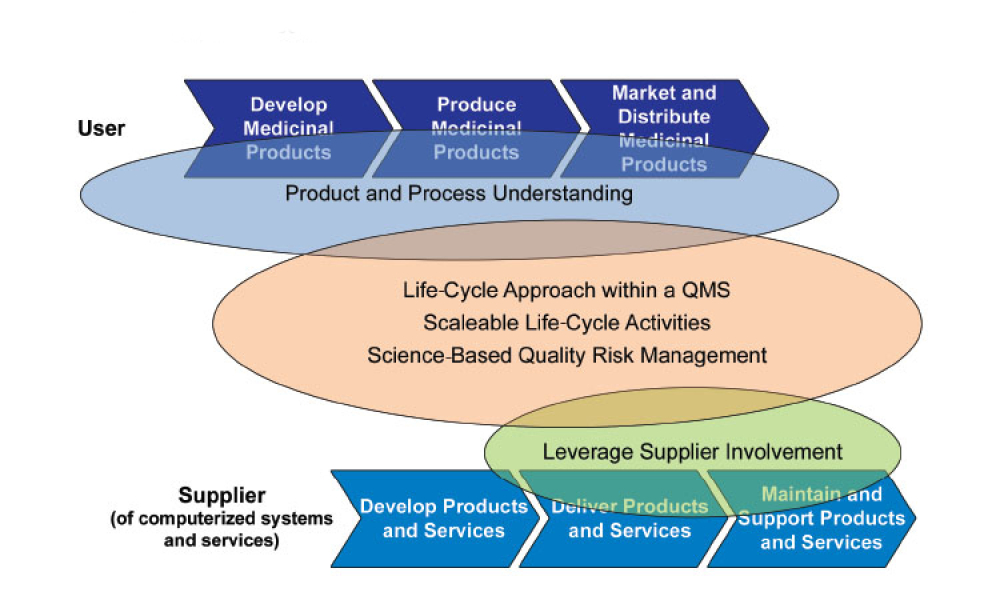
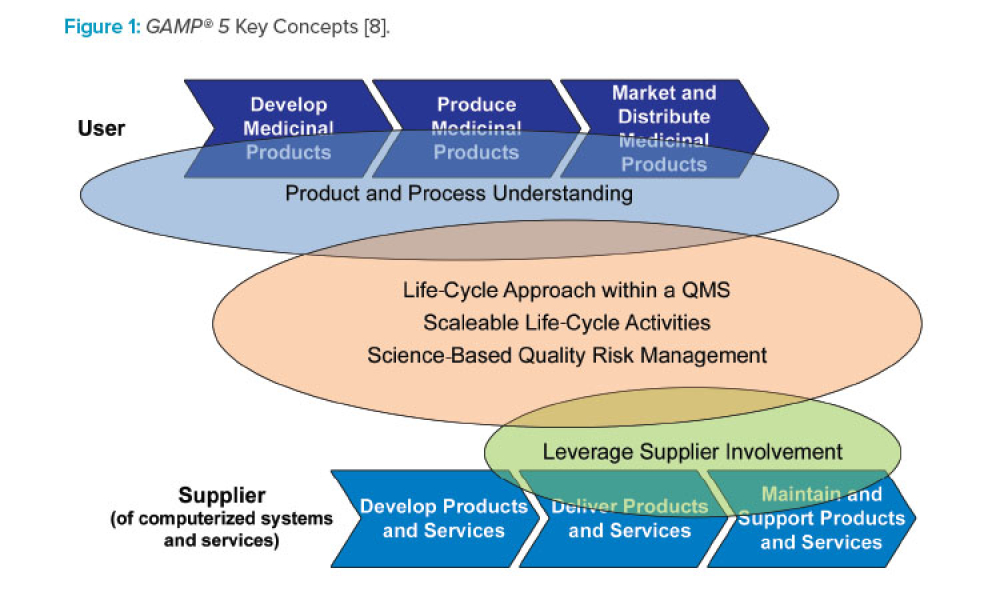
This article focuses on pragmatic quality- and risk-based approaches to IT infrastructure. It covers recommendations made by a US FDA/industry team linked to the US FDA Center for Devices and Radiological Health (CDRH) Case for Quality initiative1
- 1US Food and Drug Administration Center for Devices and Radiological Health. “Case for Quality.” 29 July 2020.