The ISPE GAMP® 5 Guide (Second Edition) was published on 29 July 2022. It was presented and discussed at the 2023 ISPE Europe Annual Conference, the 2023 ISPE Annual Meeting & Expo, and at several local...
Frank Henrichmann

Related Articles
Due to the growing digitalization of the industry, we are highly dependent on information technology (IT) systems and data. The basic ability to execute our pharmaceutical business and decision-making processes relies on the permanent availability of these IT systems and data to ensure compliance and efficiency of our business operations. But numerous factors—including criminal activities,...
GAMP® 5 (Second Edition) was published on 29th July 2022 and was presented and discussed at the 2023 ISPE Annual Meeting and at several local...
Real-world evidence (RWE) is clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of real-world data (RWD) relating to patient health status and the healthcare delivery.1
- 1US Food and Drug Administration. “Use of Real-World Evidence to Support Regulatory Decision-Making for Medical Devices: Guidance for Industry and Food...
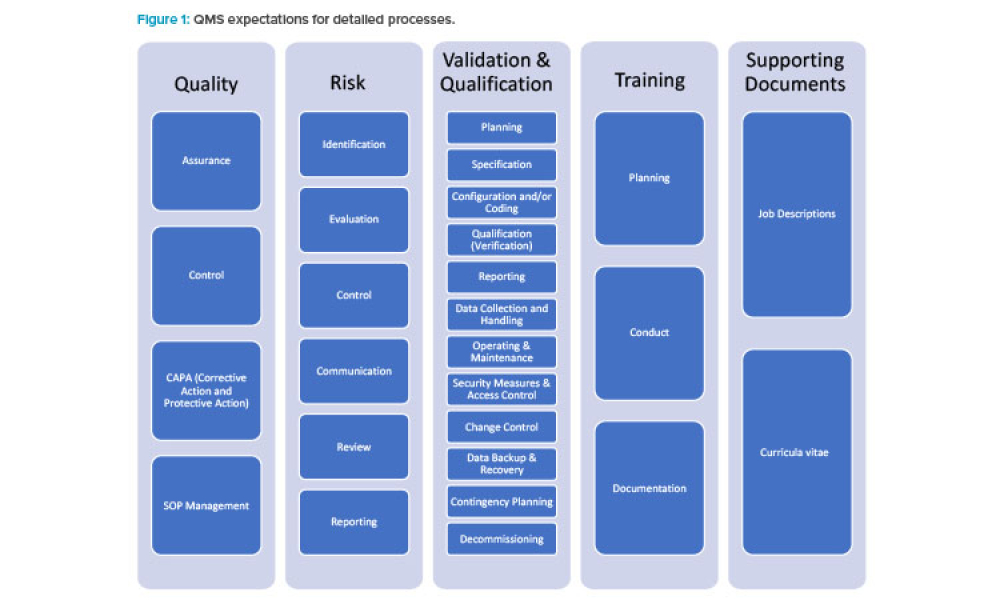
Existing risk-based approaches to computerized system compliance and validation as outlined in GAMP® 51 are applicable to a variety of life sciences organizations...
- 1International Society for Pharmaceutical Engineering. GAMP® 5 Guide: A Risk-Based Approach to Compliant GxP Computerized Systems. North Bethesda, MD: International Society for Pharmaceutical Engineering, 2008.