This blog is a companion to ISPE GAMP® 5: A Risk-Based Approach to Compliant GxP Computerized Systems (Second Edition) and the ISPE GAMP® Good Practice Guide: Manufacturing Execution Systems - A Strategic and Program Management Approach.
Pharma 4.0™
Pharma 4.0™ is the life sciences implementation of Industry 4.0, also referred to as “the 4th industrial revolution.” Each iteration of industry advancement reduces some amount of unskilled labor while creating other more technical human activities with the expectation of more efficient, accurate and humanly safe operations and products. Briefly, these are
Industry 1.0 – the age of steam for manufacturing and transportation
Industry 2.0 – more sophisticated manufacturing machinery and introduction of electric power
Industry 3.0 – introduction and advancement of computers and digital controls along with interconnected devices and the internet driving more accurate and efficient manufacturing and advanced data generation/analysis
Industry 4.0 - new technologies are connecting personnel, machines and devices more intimately creating an integrated digital and physical ecosystem including:
- sophisticated robotics
- highly advanced data analysis and artificial intelligence (AI)
- cloud-based systems
- Internet of Things (IoT) with widely connected devices/sensors, and
- augmented/virtual reality environments for training and operations.
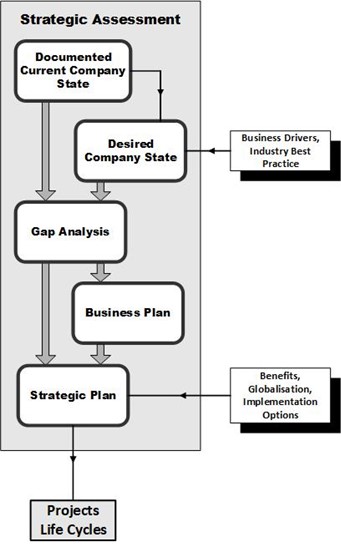
The life sciences regulatory and guidance environment is evolving as industry moves into the Pharma 4.0™ paradigm. Revisions to ISPE GAMP® 5 and related good practice guidance publications are aimed at providing support and flexibility in the design and implementation of computerized systems at all stages of life sciences product invention, development, and production.
It is also important to note that “Pharma 4.0™” applies to all life sciences products including pharmaceuticals, medical devices, and hybrid products, as well as equipment and systems facilitating development and production of activities.
The GAMP® 5 key concepts provide the framework for adhering to regulated activities:
- Product and Process Understanding
- Life Cycle Approach within a QMS
- Scalable Life Cycle Activities
- Science-Based Quality Risk Management
- Leveraging Supplier Involvement
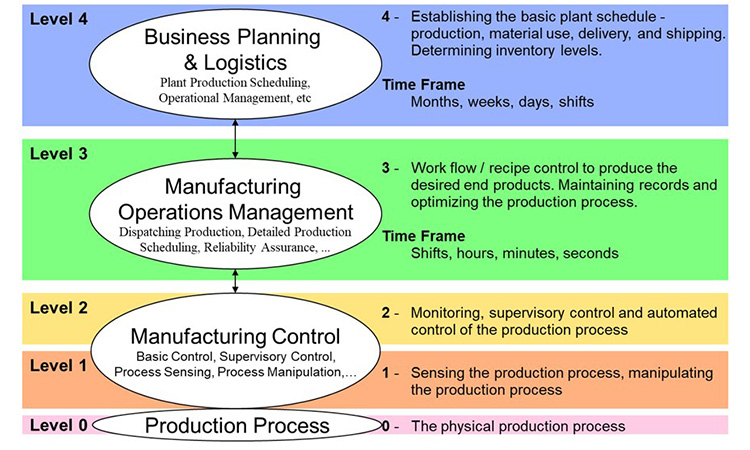
Manufacturing Execution Systems inherently utilize and create electronic records throughout manufacturing and thus are a key component of enhancing accuracy, timeliness, and overall availability of data. GAMP® 5 Second Edition contains updates to Appendix S2 Electronic Production Records in this regard.
Your Company and MES
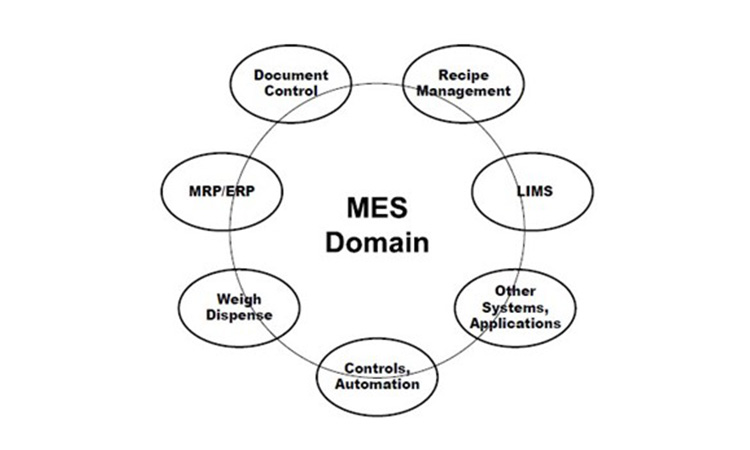
Manufacturing execution involves functionality across the enterprise, from raw material specification, quality evaluation and status, inventory, and usage (traceability) through intermediates and products involving Enterprise Resource Systems (ERP), specification systems, Laboratory Information Management Systems (LIMS), Manufacturing Control, Automation and more. Manufacturing execution can be looked at as the sum or Domain of functions needed for managing all related operations.