
Featured in this edition of iSpeak Reading Roundup, are the top blog posts from November 2019. Discover key insights for cleaning validation practices, risk-based approaches to quality, and more for what the pharmaceutical industry was reading last month.
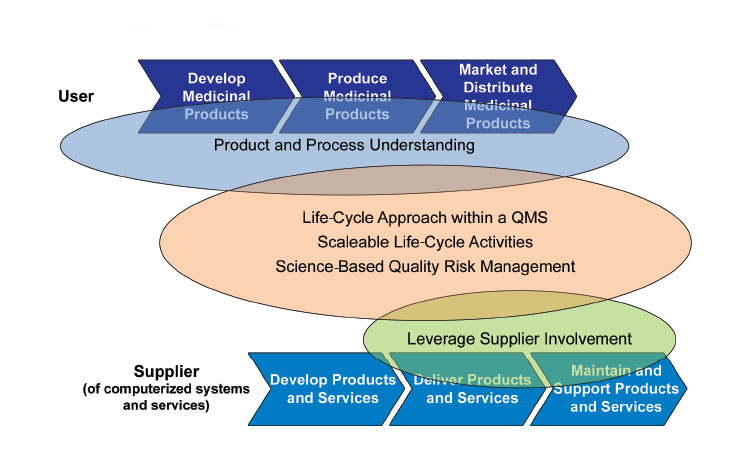
The GAMP® Global Leadership strongly supports a patient-centric and quality risk-based approach to the assurance of computerized systems. Explore the incorporation of innovative computerized technologies and approaches that advance pharmaceutical production to support product quality, patient safety, and data integrity.

See the ICH Q12 regulatory guideline, featuring detailed instruction for the break-down of complex regulation and other challenges facing the pharmaceutical industry. Professionals can adjust current production and develop new operations for excellence with the support of these management strategies.

Discover the most common violations to avoid for the implementation of a cleaning validation program in today’s industry with product-specific operations, sterile manufacturing, and how to organize pre-approved strategies. In addition, solutions to these common pitfalls are featured in this article.

Kerren Bergman, Vice President US Operations and Global HR for Hyde Engineering, discusses a crucial reminder for women to stop apologizing and be more confident in their opinions. She examines how to build your personal power, and fully understand the impact of your words in professional settings. Learn how to strengthen your voice and communicate your valuable opinions!

Read highlights from the 2019 ISPE Annual Meeting and Expo where the 2020-2022 Strategic Plan was introduced, and Frances Zipp was honored as the New chair for the ISPE International Board of Directors. See details for each award presented and the influential leaders in today’s pharmaceutical industry, who are included in the growing ISPE network.
The following blog post was provided by Peyton Myers, an undergraduate student at Appalachian State University. Myers attended the 2023 ISPE Annual Meeting & Expo in Las Vegas as an ISPE Foundation Professional Development Grant recipient.
The integration of data science in biopharmaceutical manufacturing, emphasizing data quality, tech transfer efficiency, and process optimization, is the heart of this track. Led by industry experts, discussions explore leveraging digital twins, predictive analytics, and continuous improvement initiatives. Additionally, interactive roundtable discussions provide attendees with a dynamic forum...
The pharmaceutical sector stands at a crossroads of immense possibilities. We are witnessing an unprecedented surge in innovation, fueled by a remarkable partnership between industry and regulatory authorities. For example, there are initiatives fostered by the US Food and Drug Administration (US FDA) to promote innovation with programs such as CATT (CBER Advanced Technologies Team) and ETP...