Life Cycle Assessment Analysis of Pharma Production Facility

Environmental Footprints of a Flexible Pharmaceutical Production Facility: A Life Cycle Assessment Analysis Part 1
This article was published in the September/October 2016 edition of Pharmaceutical Engineering® Magazine. In recent years, the industry has been experiencing a noticeable surge in the demand for manufacturing facilities designed to be more resource and energy efficient. Fueled by green policies, the growing environmental consciousness of stakeholders, or the sheer awareness that it is good for business, companies continue to push to enhance their sustainability performance. It has become evident that sustainability has reached mainstream and is here to stay. Attaining a better understanding of the key factors responsible for the environmental impacts of a manufacturing facility, therefore, becomes increasingly relevant. This is particularly important to target sustainability efforts effectively. In this case study, a holistic approach is applied to capture and analyze the impact of a biopharmaceutical production facility in its entirety. Therefore, the whole life cycle—from construction to operation and demolition—is examined using life cycle assessment (LCA).1 ,2 Impacts that occur directly onsite and offsite are equally considered. These include, for instance, impacts related to the manufacturing of materials, transportation of goods, and energy used in the facility or connected with the disposal of waste. A parameterized facility model is used to simulate and compare different facility configuration production scenarios. For instance, the number of production bioreactors, their batch volume and batch frequency per year, and the facility lifetime are varied. These scenario variations enable one to determine key environmental impact drivers and acquire a better understanding of relationships between facility systems. The results indicate that the key drivers for the environmental sustainability are not necessarily found directly at the facility, but rather in the supply chain.
Model Facility and Life Cycle Assessment
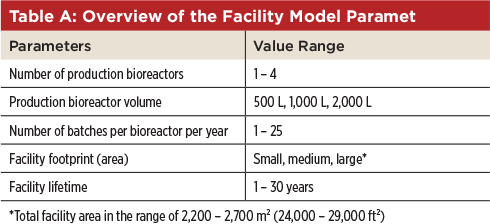
The Facility The model production facility was specifically selected to represent current trends in the biopharmaceutical industry that aim for more flexibility at a smaller scale. The facility employs exclusively single-use bioprocess systems up to a 2,000-L scale and is built up using modular construction. The production is based on a typical process for monoclonal antibodies (mAbs) with a titer of 3 g/L. China was the preferred location to be evaluated. The parameterized facility model enables analysis and comparison of various possible facility configurations. For instance, the number of production bioreactors, the process scales, or the batch frequency can be varied. Table A lists the parameters that can be modified and their value ranges considered in the facility model.
The process itself follows a generic mAb process flow. Upstream operations include media preparation, seed train, production bioreactors, and a clarification step. Downstream activities are composed of buffer preparation, capture, intermediate and polishing chromatography steps, ultrafiltration/diafiltration systems, and virus and bulk final filtration operations. The upstream and downstream process trains are operated in cleanrooms class D and class C, respectively.
Life Cycle Assessment and System Boundaries
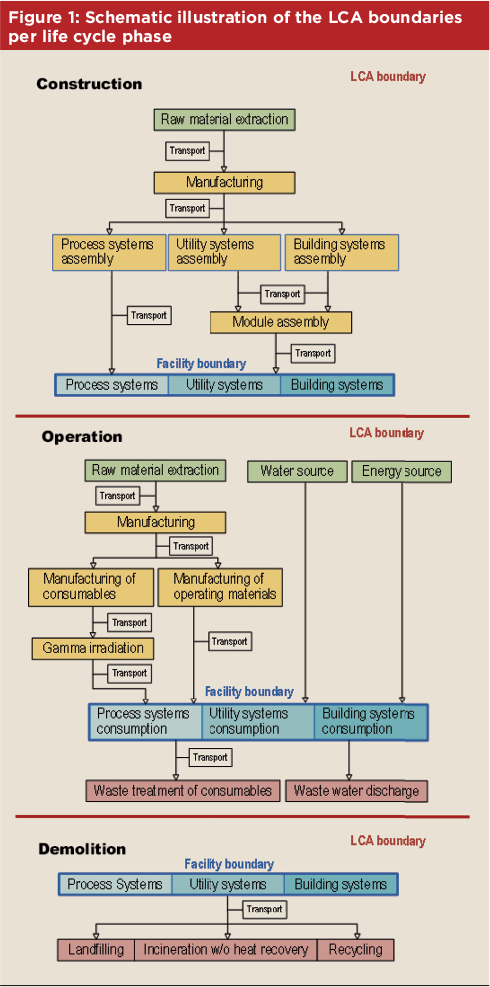
The LCA was carried out in accordance with ISO 14040 and ISO 14044 requirements. The assessment was not limited to the facility site. It considers all materials and resources utilized and waste and emissions generated worldwide as a result of the facility’s existence. The environmental impact evaluation was also not limited only to the operational phase of the facility. It included the construction and, in the supposed case, it reaches the end of life, the demolition impacts. In this way, the transportation of the facility modules during the construction phase was also considered. Moreover, the facility lifetime (i.e., years of operation) was defined as a parameter in the model. Figure 1 is a schematic illustration of the LCA boundaries for each life cycle phase. The assessment encompasses the whole facility building. This entails process, utility, and building systems:
- Process systems include all production equipment to actually run the process, from media and buffer preparation to all unit operations including production bioreactors, chromatography systems, tangential flow filtration and filtration systems, etc.
- Utility systems provide process support. It includes supply systems such as purified water and water-for-injection (WFI) generation units but also waste treatment systems such as decontamination autoclaves or neutralization systems.
- Building systems refer to the technical units that provide building services, for instance, heating, ventilation, and air conditioning (HVAC) units but also lighting and sanitary facilities. It also covers all cleanrooms (e.g., gowning, upstream and downstream process rooms, buffer and media preparation and holding, etc.) and technical areas.
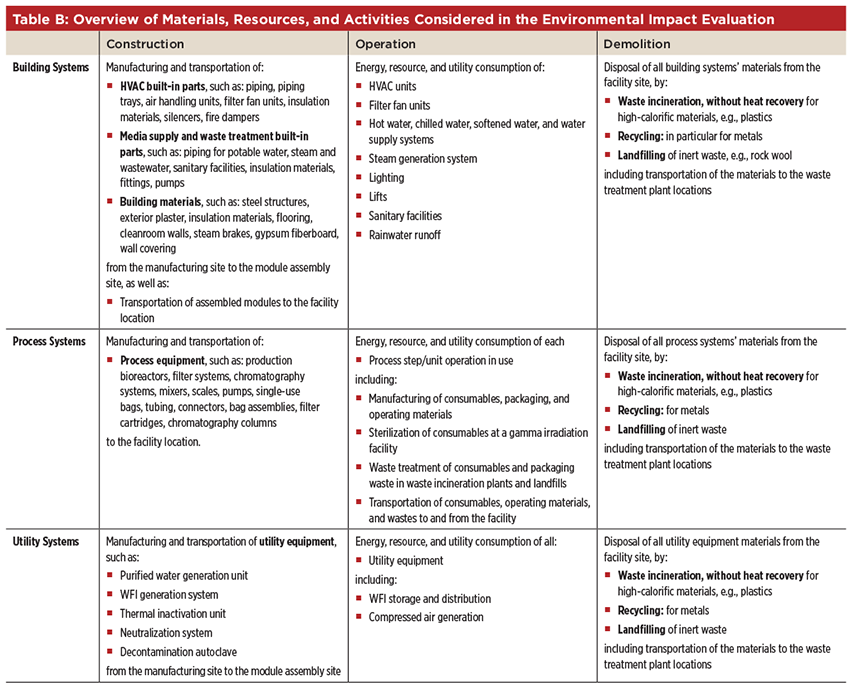
The model is further organized combining the life cycle phases with the three system types. Following this structure, a detailed overview of all material, resources, and activities considered in the environment impact evaluation is presented in Table B. This entails, for instance, the manufacturing of construction materials, process equipment or consumables, sterilization of single-use consumables in a gamma irradiation facility, and transportation of all materials to and from the site, as well as the disposal of waste in waste treatment facilities. Moreover, geographic specific factors, such as yearly energy consumption of HVAC systems, energy mixes, and industry standard waste treatment technologies3 are considered.
Pharmaceutical Engineering® Magazine: The magazine covers topics important to the global pharmaceutical industry across all sectors, including traditional pharmaceuticals, biotechnology, innovator, and generics. Pharmaceutical Engineering Magazine presents valuable information on the latest scientific and technical developments, regulatory initiatives, and innovative solutions to real-life problems and challenges through practical application articles and case studies. Stay connected with the pharmaceutical industry by becoming a member of ISPE and receive Pharmaceutical Engineering Magazine delivered to your inbox and by mail. View part two of the Environmental Footprints of a Flexible Pharmaceutical Production Facility: A Life Cycle Assessment Analysis.
- 1 International Organization for Standardization. ISO 14040: "Environmental Management—Life Cycle Assessment—Principles and Framework." Geneva, Switzerland, 2006.
- 2———. ISO 14044: "Environmental Management—Cycle Assessment—Requirements and Guidelines." Geneva, Switzerland, 2006.
- 3Wells B., et al. "Guide to Disposal of Single-Use Bioprocess Systems." BioProcess International 5, no. 8 (2007): S24–S27. http://www.bioprocessintl.com/manufacturing/supply-chain/guide-to-disposal-of-single-use-bioprocess-systems-183977




