Related Articles
Pharmaceutical manufacturing facilities produce a variety of products, including highly potent products that require safety measures to prevent adverse health effects on patients and operators. To ensure safety, these facilities use containment equipment to minimize the risk of contamination. This article presents criteria for selecting containment equipment, considering both...
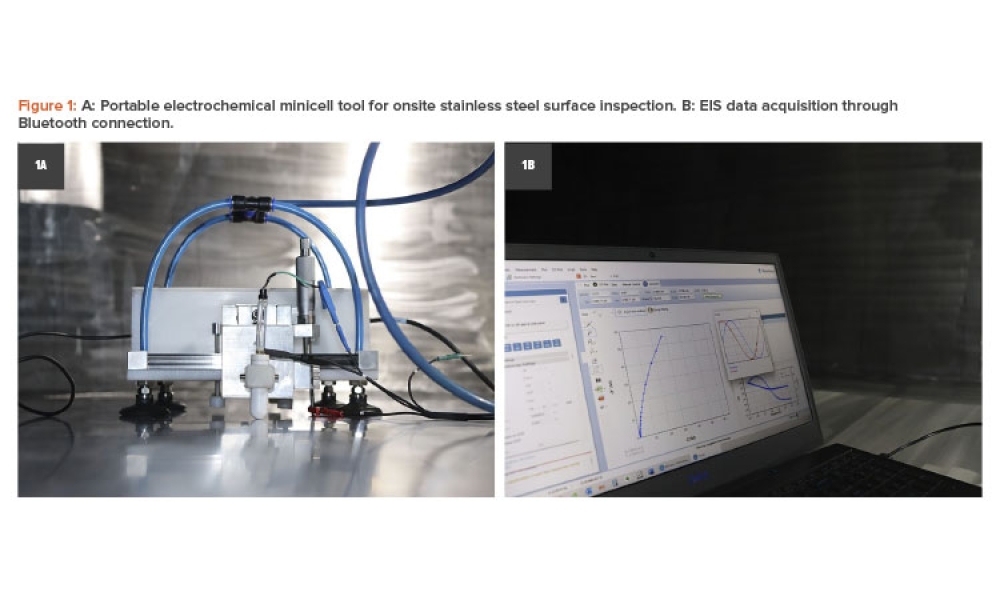
Pharmaceutical critical utilities are typically built of 316L stainless steel; nevertheless, surface degradation has been reported due to the occurrence of different phenomena. This article aims to explain how field electrochemical techniques using a portable tool can be an effective method for surface inspection, qualification, and monitoring. The surface finish assessment considered...
The new year is here and, with that, another International Women’s Day approaches! This incredibly important global movement, which takes place every 8 March, celebrates women’s achievements, raises awareness about discrimination, and advocates for accelerated equality and gender parity. ISPE’s Women in Pharma® community strives to accomplish all these goals through regional and international...